ASX/NASDAQ Announcement
HNSCC and OPMD program update
SYDNEY, April 24, 2018 /PRNewswire/ -- Benitec Biopharma Limited (ASX: BLT; NASDAQ: BNTC; NASDAQ: BNTCW) today provided an update on its two lead programs in head and neck squamous cell carcinoma (HNSCC) and oculopharyngeal muscular dystrophy (OPMD). This update should be read in conjunction with the latest corporate presentation which can be found on the Company's website.
HNSCC
Benitec is developing BB-401 as a treatment for HNSCC. BB-401 is a recombinant DNA construct that produces an antisense RNA with specificity against Epidermal Growth Factor Receptor (EGFR), an oncogenic factor overexpressed in more than 90% in lesions from patients with HNSCC. The goal of BB-401 treatment is to inhibit the expression of EGFR in the treated lesions and thus control the progression of disease and increase patient survival.
Manufacturing:
Manufacturing of BB-401 clinical product was completed at the end of last year and it is now ready for clinical use.
Clinical and Regulatory:
BB-401 was originally developed through early stage studies at the University of Pittsburgh and has been in-licensed by Benitec. In prior clinical studies conducted under an investigator-sponsored Investigational New Drug Application (IND) with the US Food & Drug Administration, BB-401 demonstrated potent activity either as a monotherapy in a dose range study or when used in combination with cetuximab and radiation. The goal of the current Phase 2 study is to confirm the clinical activity of high doses of BB-401 against HNSCC lesions.
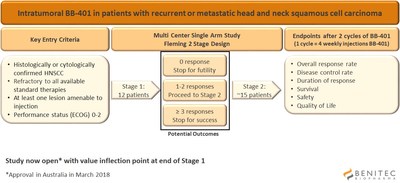
The Company continues to make good progress with the initiation of this Phase 2 clinical study which is designed as an open label study to explore the safety, tolerability and efficacy of BB-401 following intratumoral injections. These are patients who are refractory to all standard therapies such as surgery, chemotherapy and immunotherapy. The study is expected to enrol up to 30 patients at 5-8 sites across Australia and Russia. The trial is registered on www.clinicaltrials.gov with the identifier: NCT03433027, where more details can be found.
The first clinical site has now been opened in Australia and the Company anticipates having additional Australian sites open later this month. Regulatory review with the Ministry of Health is ongoing in Russia and the Company expects approval at the end of May.
As shown in the figure below, the primary outcome is the objective response rate to BB-401 in the injected lesion. Additional secondary endpoints include progression free survival, overall survival, duration of response, disease control rate, safety and tolerability. The study has a two-stage design which allows for stopping of the study based on either success or futility at the end of the first stage, after 12 patients have been enrolled and monitored through the primary outcome measure. This interim analysis is anticipated to occur around the end of the 2018 calendar year.
Market Expansion Opportunities:
In addition to HNSCC, EGFR overexpression has been associated with a number of cancers, including epithelial tumors of the head and neck, squamous cell cancers of the lungs, anal cancers, and glioblastoma. As such, Benitec is exploring other potential clinical indications for anti-EGFR strategies, including rare cancers.
OPMD
BB-301 is a single vector (gene therapy construct) system that uses DNA directed RNA interference (ddRNAi) to silence expression of the mutant gene associated with OPMD, while simultaneously expressing a copy of the normal, healthy version of the same gene to restore the function of that gene.
Clinical:
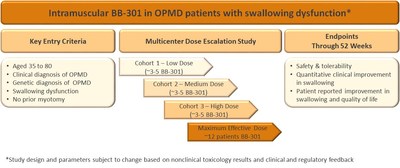
BB-301 is being developed as a treatment for OPMD. The first clinical study of BB-301 will be focused on treating dysphagia in patients with OPMD. Defined as a difficulty in swallowing, it is the inability to restrict food intake into the stomach versus being aspirated into the airways in dysphagia which causes the majority of serious health problems for patients with OPMD including death. In the initial clinical study, BB-301 will be administered via intramuscular injection into the cricopharyngeus, a throat muscle which regulates the passage of swallowed food into the oesophagus. The endpoints of this study are designed to monitor safety as well as determine if treatment results in the improvement of swallowing function and improvement in swallowing quality of life in OPMD patients.
The clinical protocol has received feedback from regulatory agencies from the US (FDA), Canada (Health Canada) and several agencies within the EU. Further refinement of the protocol is anticipated in a face-to-face Clinical Advisory Board meeting with our Key Opinion Leaders including doctors who managed treatment options for OPMD patients as well as experts in the quantitative assessment of dysphagia.
With only palliative care and no marketed therapeutics available, there has been a significant level of enthusiasm and hope from the patients in the OPMD community for BB-301. Given this and given the known geographical clusters of OPMD, Benitec is optimistic about patient accessibility when the IND, or other initial regulatory application, has been filed and approved.
Manufacturing
BB-301 is being produced using baculovirus-based technology, a highly scalable methodology that permits high yield, cost efficient production of the BB-301 product. The Company has successfully produced high titer and highly active material at the 50L scale which is being used for the ongoing toxicology studies. The focus has shifted to producing supplies of BB-301 at a 250L scale, which will support the clinical program.
Toxicology:
Before BB-301 can be tested in human clinical trials, the necessary safety studies (toxicology studies) must be performed in large animal models. These IND-enabling toxicology studies are to ensure that the delivery procedures as well as the doses of BB-301 that we intend to use do not cause any obvious safety problems.
The IND-enabling toxicology studies with BB-301 are being conducted in sheep. This animal model was selected because the weight of the sheep and the size of key muscles in the upper digestive system are consistent with human subjects. These features are key to support the route of administration of direct injection into cricopharyngeal muscle. Given the complexity of the intramuscular route of administration in the sheep and input from the regulatory agencies, the number of animals being used in these studies has been increased resulting in slightly slower timelines. The first toxicology studies are now underway and the Company anticipates filing the IND in 1Q 2019.
Regulatory:
As noted above, regulatory discussions have been completed with agencies in the United States, Canada and Europe. These are all regions with key OPMD patient clusters and the purpose of these meetings was to discuss the regulatory development pathway for BB-301 as a treatment for OPMD and to ensure that Benitec's proposed development program which includes the existing safety and efficacy data, manufacturing plans, nonclinical plans and clinical study design addresses the regulatory expectations of these agencies.
Benitec's SME (Small and Medium Sized Business Entities) status in the European Union combined with the Orphan Drug Designation in the US and EU are invaluable as Benitec progresses BB-301 towards commercialisation and paves the way for a potentially clear and expeditious development pathway.
Market Expansion Opportunities:
BB-301 is initially being developed as an intramuscular injection to treat the dysphagia associated with OPMD. Assuming success, there is the potential to treat earlier stages of dysphagia, systemic administration to treat proximal muscle weakness and ptosis and prophylactic treatment to prevent the development of muscle weakness.
In addition to the market expansion opportunities noted above for BB-301 there are opportunities to use single vector 'silence and replace' strategies in other disease indications, particularly rare diseases, as a competitive advantage versus other companies and technologies.
Commenting on the update CEO Greg West said: "The advancement of the HNSCC program into the clinic and the OPMD program becoming closer to 'clinic ready' shows how our novel technology can address significant unmet clinical needs. We are very pleased with the progress in these programs. Positive outcomes in the clinical trials would contribute to both the validation of our technology and improvement in the valuation of Benitec.
For further information regarding Benitec and its activities, please contact the persons below, or visit the Benitec website at www.benitec.com
|
Australia Investor Relations |
United States Investor Relations |
|
Market Eye Orla Keegan Director Tel: +61 (2) 8097 1201 Email: orla.keegan@marketeye.com.au |
M Group Strategic Communications |
About Benitec Biopharma Limited:
Benitec Biopharma Limited (ASX: BLT; NASDAQ: BNTC; NASDAQ: BNTCW) is a biotechnology company developing innovative therapeutics based on its patented gene-silencing technology called ddRNAi or 'expressed RNAi'. Based in Sydney, Australia with laboratories in Hayward, California (USA), and collaborators and licensees around the world, the company is developing ddRNAi-based therapeutics for chronic and life-threatening human conditions including head & neck squamous cell carcinoma, OPMD retinal based diseases such as wet age-related macular degeneration, and hepatitis B. Benitec has also licensed ddRNAi to other biopharmaceutical companies for applications including HIV/AIDS, Huntington's Disease, chronic neuropathic pain, cancer immunotherapy and retinitis pigmentosa.
About OPMD:
OPMD is a rare inherited myopathy characterized by dysphagia (difficulty in swallowing), the loss of muscle strength, and weakness in multiple parts of the body. Patients typically suffer from severe dysphagia, ptosis (eye lid drooping), tongue atrophy, proximal lower limb weakness, dysphonia (altered and weak voice), limitation in looking upward, as well as facial muscle and proximal upper limb weakness. Progressing throughout that patient's life, OPMD is not typically diagnosed until the individuals reach their late 40s. As the dysphagia becomes more severe, patients become malnourished, lose significant weight, become dehydrated and suffer from repeated incidents of aspiration pneumonia. The last two symptoms are often the cause of death. No cure is currently available for OPMD. The cricopharyngeal myotomy is the only treatment available to improve swallowing in these patients, but because the root cause of the genetic disease has not been addressed, the pharyngeal musculature still undergoes progressive degradation leading to the previously mentioned complications.
About Head and Neck Cancer:
Cancers that are known as head and neck cancers usually begin in the squamous cells that line the moist mucosal surfaces inside the head and neck, such as inside the mouth and the throat. The global incidence of HNSCC is expected to increase from approximately 119,000 cases in 2016 to over 136, 000 cases in 2026. Head and neck cancers are more than twice as common among men as they are among women. Squamous cell carcinoma of the head and neck accounts for more than 90% of all head and neck cancers, and more than 50% of HNSCC patients present with Stage III or higher disease (locally advanced or metastatic), which has higher potential for progression and recurrence. For patients with recurrent of metastatic HNSCC the median overall survival is 7.8 months and the five year survival rate is 3.6%. Total drug sales in the HNSCC markets in the seven major markets (United States, France, Germany, Italy, Spain, United Kingdom and Japan) are expected to increase from $579.4 million in 2016 to just over $4.1 billion in 2026, at a Compound Annual Growth Rate (CAGR) of 21.6%. Reference: GlobalData Report (March 2018): Head and Neck Squamous Cell Carcinoma – Opportunity Analysis and Forecast to 2026
Safe Harbor Statement:
This press release contains "forward-looking statements" within the meaning of section 27A of the US Securities Act of 1933 and section 21E of the US Securities Exchange Act of 1934. Any forward-looking statements that may be in this ASX/Nasdaq announcement are subject to risks and uncertainties relating to the difficulties in Benitec's plans to develop and commercialise its product candidates, the timing of the initiation and completion of preclinical and clinical trials, the timing of patient enrolment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and Benitec's product candidates, potential future out-licenses and collaborations, the intellectual property position and the ability to procure additional sources of financing. Accordingly, you should not rely on those forward-looking statements as a prediction of actual future results.
![]() View original content with multimedia:http://www.prnewswire.com/news-releases/asxnasdaq-announcement-300635074.html
View original content with multimedia:http://www.prnewswire.com/news-releases/asxnasdaq-announcement-300635074.html
SOURCE Benitec Biopharma Limited
Released April 24, 2018