10-Q: Quarterly report pursuant to Section 13 or 15(d)
Published on May 16, 2022
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2022
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from
to
Commission File Number 001-39267
(Exact name of registrant as specified in its charter)
-0206 |
||
|
(State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
(Address of principal executive offices & zip code)
(510 ) 780-0819
(Registrant’s telephone number including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
||
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation Yes ☒ No ☐
S-T
(§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated
filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2
of the Exchange Act. | Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
Non-accelerated filer |
☒ | Smaller reporting company | ||||
| Emerging growth company | ||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule ☒
12b-2
of the Exchange Act). Yes ☐ or No We had 8,171,690 shares of our common stock outstanding as of the close of business on May
16
, 2022. BENITEC BIOPHARMA INC.
INDEX TO FORM
10-Q
| 2 | ||||||
| 3 | ||||||
| 3 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 7 | ||||||
| 8 | ||||||
| 17 | ||||||
| 32 | ||||||
| 32 | ||||||
| 33 | ||||||
| 33 | ||||||
| 33 | ||||||
| 33 | ||||||
| 33 | ||||||
| 33 | ||||||
| 33 | ||||||
| 34 | ||||||
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Report contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. Our forward-looking statements relate to future events or our future performance and include, but are not limited to, statements concerning our business strategy, future commercial revenues, market growth, capital requirements, new product introductions, expansion plans and the adequacy of our funding. All statements, other than statements of historical fact included in this Report, are forward-looking statements. When used in this Report, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
Some of the risks and uncertainties that may cause our actual results, performance or achievements to differ materially from those expressed or implied by forward-looking statements include the following:
| • | the success of our plans to develop and potentially commercialize our product candidates; |
| • | the timing of the initiation and completion of preclinical studies and clinical trials; |
| • | the timing and sufficiency of patient enrollment and dosing in any future clinical trials; |
| • | the timing of the availability of data from clinical trials; |
| • | the timing and outcome of regulatory filings and approvals; |
| • | unanticipated delays; |
| • | sales, marketing, manufacturing and distribution requirements; |
| • | market competition and the acceptance of our products in the marketplace; |
| • | regulatory developments in the United States of America; |
| • | the development of novel AAV vectors; |
| • | the plans of licensees of our technology; |
| • | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; |
| • | our dependence on our relationships with collaborators and other third parties; |
| • | expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors, including our capital structure; |
| • | the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; |
| • | our intellectual property position and the duration of our patent portfolio; |
| • | the impact of local, regional, and national and international economic conditions and events; and |
| • | the impact of the current COVID-19 pandemic, the disease caused by the SARS-CoV-2 |
as well as other risks detailed under the caption “Risk Factors” in this Report and in other reports filed with the SEC. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Report, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain. We have based the forward-looking statements included in this Report on information available to us on the date of this Report or on the date thereof. Except as required by law we undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised to consult any additional disclosures that we may make directly to you or through reports that we, in the future, may file with the SEC, including annual reports on Form
10-K,
quarterly reports on Form 10-Q
and current reports on Form 8-K.
All forward-looking statements included herein or in documents incorporated herein by reference are expressly qualified in their entirety by the cautionary statements contained or referred to elsewhere in this Report.
2
PART I—FINANCIAL INFORMATION
ITEM 1. Financial Statements
BENITEC BIOPHARMA INC.
Consolidated Balance Sheets
(in thousands, except par value and share amounts)
March 31, |
June 30, |
|||||||
2022 |
2021 |
|||||||
(Unaudited) |
||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | $ | ||||||
| Trade and other receivables |
||||||||
| Prepaid and other assets |
||||||||
| |
|
|
|
|||||
| Total current assets |
||||||||
| Property and equipment, net |
||||||||
| Deposits |
||||||||
| Other assets |
||||||||
| Right-of-use |
||||||||
| |
|
|
|
|||||
| Total assets |
$ | $ | ||||||
| |
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Trade and other payables |
$ | $ | ||||||
| Accrued employee benefits |
||||||||
| Lease liabilities, current portion |
||||||||
| |
|
|
|
|||||
| Total current liabilities |
||||||||
| Lease liabilities, less current portion |
|
|||||||
| |
|
|
|
|||||
| Total liabilities |
||||||||
| |
|
|
|
|||||
| Commitments and contingencies (Note 10) |
|
|
||||||
| Stockholders’ equity: |
||||||||
| Common stock, $ value- shares authorized; |
||||||||
| Additional paid-in capital |
||||||||
| Accumulated deficit |
( |
) | ( |
) | ||||
| Accumulated other comprehensive loss |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Total stockholders’ equity |
||||||||
| |
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | $ | ||||||
| |
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
3
BENITEC BIOPHARMA INC.
Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(in thousands, except share and per share amounts)
Three Months Ended |
Nine Months Ended |
|||||||||||||||
March 31, |
March 31, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
| Revenue: |
||||||||||||||||
| Licensing revenues from customers |
$ | $ | $ | $ | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total revenues |
||||||||||||||||
| Operating expenses |
||||||||||||||||
| Royalties and license fees |
|
|
||||||||||||||
| Research and development |
||||||||||||||||
| General and administrative |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| Other income (loss): |
||||||||||||||||
| Foreign currency transaction gain (loss) |
( |
) | ( |
) | ||||||||||||
| Interest expense, net |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| Other income (expense), net |
( |
) |
|
( |
) | |||||||||||
| Unrealized loss on investment |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total other income (loss), net |
( |
) | ( |
) | ( |
) | ||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| Other comprehensive income: |
||||||||||||||||
| Unrealized foreign currency translation (loss) gain |
( |
) | ( |
) | ( |
) | ||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total other comprehensive (loss) income |
( |
) | ( |
) | ( |
) | ||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total comprehensive loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss per share: |
||||||||||||||||
| Basic and diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| Weighted average number of shares outstanding: basic and diluted |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these consolidated financial statements.
4
BENITEC BIOPHARMA INC.
Consolidated Statements of Stockholders’ Equity
(Unaudited)
(in thousands, except share amounts)
Common Stock |
Additional Paid-in
Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Total Stockholders’ Equity |
||||||||||||||||||||
Shares |
Amount |
|||||||||||||||||||||||
| Balance at June 30, 2020 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
| Share-based compensation |
— | — | — | — | ||||||||||||||||||||
| Forfeiture of share-based payments |
— | — | ( |
) | — | — | ||||||||||||||||||
| Foreign currency translation gain |
— | — | — | — | ||||||||||||||||||||
| Net loss |
— | — | — | ( |
) | ( |
) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2020 |
( |
) | ( |
) | ||||||||||||||||||||
| Issuance of common stock and pre-funded warrants sold for cash, net of issuance costs of $ |
— | — | ||||||||||||||||||||||
| Exercise of pre-funded warrants |
— | — | — | — | — | |||||||||||||||||||
| Share-based compensation |
— | — | — | — | ||||||||||||||||||||
| Forfeiture of share-based payments |
— | — | ( |
) | — | — | ||||||||||||||||||
| Foreign currency translation gain |
— | — | — | — | ||||||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2020 |
( |
) | ( |
) | ||||||||||||||||||||
| Exercise of pre-funded warrants |
— | — | ||||||||||||||||||||||
| Share-based compensation |
— | — | — | — | ||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at March 31, 2021 |
$ | $ | |
$ | ( |
) | $ | ( |
) | $ | |
|||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
5
BENITEC BIOPHARMA INC.
Consolidated Statements of Stockholders’ Equity
(Unaudited)
(in thousands, except share amounts)
Common Stock |
Additional Paid-in
Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Total Stockholders’ Equity |
||||||||||||||||||||
Shares |
Amount |
|||||||||||||||||||||||
| Balance at June 30, 2021 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | |||||||||||||||
| Share-based compensation |
— | — | — | — | ||||||||||||||||||||
| Foreign currency translation gain |
— | — | — | — | ||||||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 30, 2021 |
( |
) | ( |
) | ||||||||||||||||||||
| Share-based compensation |
— | — | — | — | ||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2021 |
( |
) | ( |
) | ||||||||||||||||||||
| Share-based compensation |
— | — | — | |||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
| Net loss |
— | — | — | ( |
) | ( |
) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at March 31, 2022 |
$ | $ | |
$ | ( |
) | $ | ( |
) | $ | ||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
6
BENITEC BIOPHARMA INC.
Consolidated Statements of Cash Flows
(Unaudited)
(in thousands)
Nine Months Ended |
||||||||
March 31, |
||||||||
2022 |
2021 |
|||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
||||||||
| Amortization of right-of-use |
||||||||
| Unrealized loss on investment |
||||||||
| Share-based compensation expense |
||||||||
| Changes in operating assets and liabilities: |
||||||||
| Trade and other receivables |
||||||||
| Other assets |
||||||||
| Trade and other payables |
||||||||
| Accrued employee benefits |
||||||||
| Lease liabilities |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Net cash used in operating activities |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Purchases of property and equipment |
|
( |
) | |||||
| |
|
|
|
|||||
| Net cash used in investing activities |
|
( |
) | |||||
| |
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from issues of shares and pre-funded warrants |
|
|||||||
| Shares and pre-funded warrant issuance costs |
|
( |
) | |||||
| |
|
|
|
|||||
| Net cash provided by financing activities |
|
|||||||
| |
|
|
|
|||||
| Effects of exchange rate changes on cash and cash equivalents |
( |
) | ||||||
| |
|
|
|
|||||
| Net increase (decrease) in cash and cash equivalents |
( |
) | ||||||
| Cash and cash equivalents, beginning of period |
||||||||
| |
|
|
|
|||||
| Cash and cash equivalents, end of period |
$ | $ | ||||||
| |
|
|
|
|||||
| Supplemental disclosure of cash flow information: |
||||||||
| Re-measurement of operating lease right-of-use |
$ | $ | |
|||||
| |
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
7
BENITEC BIOPHARMA INC.
Notes to Consolidated Financial Statements
(Unaudited)
1. Business
Benitec Biopharma Inc. (the “Company”) is a corporation formed under the laws of Delaware, United States of America, on November 22, 2019 and listed on the Nasdaq Capital Market (“Nasdaq”) under the symbol “BNTC”. Benitec Biopharma Inc. is the parent entity of a number of subsidiaries including the previous parent entity Benitec Biopharma Limited (“BBL”). BBL was incorporated under the laws of Australia in 1995 and was listed on the Australian Securities Exchange, or ASX, from 1997 until April 15, 2020. On August 14, 2020, BBL reorganized as a Proprietary Limited company and changed its name to Benitec Biopharma Proprietary Limited. The Company’s business focuses on the development of novel genetic medicines. Our proprietary platform, called
DNA-directed
RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes. During the year ended June 30, 2021, the Company completed an organization restructuring as part of the commercial desire to provide a more efficient structure for the future as the Company transitioned its operations to the
United State
s.
The Company’s fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year end June 30 of that calendar year.
The consolidated financial statements of Benitec Biopharma Inc. are presented in United States dollars and consist of Benitec Biopharma Inc. and its wholly owned subsidiaries:
Principal place of business/country of incorporation | ||
| Benitec Biopharma Proprietary Limited (“BBL”) |
||
| Benitec Australia Proprietary Limited |
||
| Benitec Limited |
||
| Benitec, Inc. |
||
| Benitec LLC |
||
| RNAi Therapeutics, Inc. |
||
| Tacere Therapeutics, Inc. |
||
| Benitec IP Holdings, Inc. |
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation
The Company’s consolidated financial statements contained in this Quarterly Report on Form
Accordingly, certain information and disclosures required by GAAP for annual financial statements have been omitted. In the opinion of management, all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation have been included. Interim financial results are not necessarily indicative of results anticipated for the full year. These consolidated financial statements should be read in conjunction with the Company’s audited financial statements and accompanying notes included in the Company’s Annual Report on Form
10-Q
have been prepared in accordance with generally accepted accounting principles in the U.S. (“GAAP”) for interim financial information and with the instructions to Form 10-Q
and Article 8 of U.S. Securities and Exchange Commission (“SEC”) Regulation
S-X.
10-K
for the year ended June 30, 2021. Reference is frequently made herein to the Financial Accounting Standards Board (the “FASB”) Accounting Standards Codification (“ASC”). This is the source of authoritative GAAP recognized by the FASB to be applied to
non-governmental
entities. Principles of Consolidation
The consolidated financial statements include the Company’s accounts and the accounts of its wholly-owned subsidiaries. All intercompany transactions and balances have been eliminated.
Use of Estimates
The preparation of the Company’s consolidated financial statements requires management to make estimates and assumptions that impact the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements and accompanying notes. The most significant estimates and assumptions in the Company’s
8
consolidated financial statements include the estimates of useful lives of property and equipment, valuation of the operating lease liability and related asset, allowance for uncollectable receivables, foreign currency translation due to certain average exchange rates applied in lieu of spot rates on transaction dates, and accrued research and development expenses. These estimates and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s future results of operations will be affected.
right-of-use
Risks and Uncertainties
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, protection of proprietary technology, dependence on key personnel, reliance on single-source vendors and collaborators, availability of raw materials, patentability of the Company’s products and processes and clinical efficacy and safety of the Company’s products under development, compliance with government regulations and the need to obtain additional financing to fund operations.
There can be no assurance that the Company’s research and development will be successfully completed, that adequate protection for the Company’s intellectual property will be obtained or maintained, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. Even if the Company’s product development efforts are successful, it is uncertain when, if ever, the Company will generate significant revenue from product sales. The Company operates in an environment of rapid technological change and substantial competition from other pharmaceutical and biotechnology companies. In addition, the Company is dependent upon the services of its employees, consultants and other third parties.
Moreover, the current
COVID-19
pandemic, which is impacting worldwide economic activity, poses risks that the Company or its employees, contractors, suppliers, and other partners may be prevented or inhibited from conducting business activities for an indefinite period of time which may delay the start-up
and conduct of the Company’s clinical trials, and negatively impact manufacturing and testing activities performed by third parties. Any significant delays may impact the use and sufficiency of the Company’s existing cash reserves, and the Company may be required to raise additional capital earlier than it had previously planned. The Company may be unable to raise additional capital if and when needed, which may result in delays or suspension of its development plans. The extent to which the pandemic will impact the Company’s business will depend on future developments that are highly uncertain and cannot be predicted at this time. Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment.
Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency and reporting currency is the United States dollar. BBL’s functional currency is the Australian dollar (AUD). Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive loss.” Gains and losses resulting from foreign currency translation are included in the consolidated statements of operations and comprehensive loss as other comprehensive income (loss).
Other comprehensive income for all periods presented includes both foreign currency translation gains and losses.
9
Fair Value Measurements
The Company measures its financial assets and liabilities in accordance with GAAP using ASC 820, For certain financial instruments, including cash and cash equivalents, accounts receivable, and accounts payable, the carrying amounts approximate fair value due to their short maturities.
Fair Value Measurements.
The Company follows accounting guidance for financial assets and liabilities. ASC 820 defines fair value, provides guidance for measuring fair value and requires certain disclosures. The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The following is a brief description of those three levels:
| Level 1: | Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities. |
| Level 2: | Inputs, other than quoted prices that are observable, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active. |
| Level 3: | Unobservable inputs in which little or no market data exists, therefore developed using estimates and assumptions developed by us, which reflect those that a market participant would use. |
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand and at banks, short-term deposits with an original maturity of three months or less with financial institutions, and bank overdrafts. Bank overdrafts are reflected as a current liability on the consolidated balance sheets. There were no cash equivalents as of March 31, 2022 and June 30, 2021.
Concentrations of Risk
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash and cash equivalents. The Company maintains deposits at federally insured financial institutions in excess of federally insured limits. The Company has not experienced any losses in such accounts, and management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
Trade and Other Receivables
As amounts become uncollectible, they will be charged to an allowance and operations in the period when a determination of collectability is made. Any estimates of potentially uncollectible customer accounts receivable will be made based on an analysis of individual customer and historical
write-off
experience. The Company’s analysis includes the age of the receivable account, creditworthiness of the customer and general economic conditions. Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. Expenditures for maintenance and repairs are expensed as incurred; additions, renewals, and improvements are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation and amortization are removed from the respective accounts, and any gain or loss is included in operations. Depreciation and amortization of property and equipment is calculated using the straight-line basis over the following estimated useful lives:
| Software |
4 | |
| Lab equipment |
7 | |
| Computer hardware |
5 | |
| Leasehold improvements |
Impairment of Long-Lived Assets
Property and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the assets. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable.
10
Trade and other payables
These amounts represent liabilities for goods and services provided to the Company prior to the end of the period and which are unpaid. Due to their short-term nature, they are measured at amortized cost and are not discounted. The amounts are unsecured and are usually paid within 30 days of recognition.
Leases
At lease commencement, the Company records a lease liability based on the present value of lease payments over the expected lease term. The Company calculates the present value of lease payments using the discount rate implicit in the lease, unless that rate cannot be readily determined. In that case, the Company uses its incremental borrowing rate, which is the rate of interest that the Company would have to pay to borrow on a collateralized basis an amount equal to the lease payments over the expected lease term. The Company records a corresponding lease asset based on the lease liability, adjusted for any lease incentives received and any initial direct costs paid to the lessor prior to the lease commencement date.
right-of-use
After lease commencement, the Company measures its leases as follows: (i) the lease liability based on the present value of the remaining lease payments using the discount rate determined at lease commencement; and (ii) the lease asset based on the remeasured lease liability, adjusted for any unamortized lease incentives received, any unamortized initial direct costs and the cumulative difference between rent expense and amounts paid under the lease agreement. Any lease incentives received and any initial direct costs are amortized on a straight-line basis over the expected lease term. Rent expense is recorded on a straight-line basis over the expected lease term.
right-of-use
Basic and Diluted Net Loss Per Share
Basic net loss per share is calculated by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is calculated by dividing net loss by the weighted-average number of common shares outstanding plus potential common shares. Stock options, warrants and convertible instruments are considered potential common shares and are included in the calculation of diluted net loss per share using the treasury stock method when their effect is dilutive. Potential common shares are excluded from the calculation of diluted net income (loss) per share when their effect is anti-dilutive. As of March 31, 2022, and June 30, 2021, there were 845,159 and 809,159 potential common shares, respectively, that were excluded from the calculation of diluted net loss per share because their effect was anti-dilutive.
Revenue Recognition
The Company recognizes revenue in accordance with that core principle by applying the following steps:
Step 1: Identify the contract(s) with a customer.
Step 2: Identify the performance obligations in the contract.
Step 3: Determine the transaction price.
Step 4: Allocate the transaction price to the performance obligations in the contract.
Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation.
The Company applies judgement in determining whether contracts entered into fall within the scope of ASC 606— (“ASC 606”). In doing so, management considers the commercial substance of the transaction and how risks and benefits of the contract accrue to the various parties to the contract.
Revenue from Contracts with Customers
Management has also made the judgement that the grant of the license and transfer of associated
know-how
and materials are accounted for as one performance obligation as they are not considered to be distinct; they are highly interrelated and could not provide benefits to the customer independently from each other. Judgements were made in relation to the transfer of the license and know-how
and whether this should be recognized over time or a point in time. The point in time has been determined with regard to the point at which the transfer of know-how
has substantially been completed and the customer has control of the asset and the ability to direct the use of and receive substantially all of the remaining benefits. Licensing revenues
Revenue from licensees of the Company’s intellectual property reflects the transfer of a right to use the intellectual property as it exists at the point in time in which the license is transferred to the customer. Consideration can be variable and is estimated using the most likely amount method and is constrained to the extent that it is probable that a significant reversal will not occur. Revenue is recognized as or when the performance obligations are satisfied.
11
The Company recognizes contract liabilities for consideration received in respect of unsatisfied performance obligations and reports these amounts as other liabilities in the consolidated balance sheet. Similarly, if the Company satisfies a performance obligation before it receives the consideration, the Company recognizes either a contract asset or a receivable in its consolidated balance sheet, depending on whether something other than the passage of time is required before the consideration is due.
Royalties
Revenue from licensees of the Company’s intellectual property reflect a right to use the intellectual property as it exists at the point in time in which the license is granted. Where consideration is based on sales of product by the licensee, revenue is recognized when the customer’s subsequent sales of products occur.
Services revenue
Revenue is earned (constrained by variable considerations) from the provision of research and development services to customers. Services revenue is recognized when performance obligations are either satisfied over time or at a point in time. Generally, the provision of research and development services under a contract with a customer will represent satisfaction of a performance obligation over time where the Company retains the right to payment for services performed but not yet completed.
Research and Development Expense
Research and development costs are expensed when incurred. Research and development expenses relate primarily to the cost of conducting clinical and
pre-clinical
trials. Pre-clinical
and clinical development costs are a significant component of research and development expenses. Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. Generally, the costs, and therefore estimates, associated with clinical trial contracts are based on the number of patients, drug administration cycles, the type of treatment and the outcome since the length of time before actual amounts can be determined will vary depending on length of the patient cycles and the timing of the invoices by the clinical trial partners. Share-based Compensation Expense
The Company records share-based compensation in accordance with ASC 718, . ASC 718 requires the fair value of all share-based compensation awarded to employees and
Stock Compensation
non-employees
to be recorded as an expense over the shorter of the service period or the vesting period. The Company determines employee and non-employee
share-based compensation based on the grant-date fair value using the Black-Scholes Option Pricing Model. Income Taxes
The Company is subject to Australia and United States income tax laws. The Company follows ASC 740 , when accounting for income taxes, which requires an asset and liability approach to financial accounting and reporting for income taxes. Deferred income tax assets and liabilities are computed annually for temporary differences between the financial statements and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount more likely than not to be realized.
Accounting for Income Taxes
For uncertain tax positions that meet a “more likely than not” threshold, the Company recognizes the benefit of uncertain tax positions in the consolidated financial statements. The Company’s practice is to recognize interest and penalties, if any, related to uncertain tax positions in income tax expense in the consolidated statements of operations.
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU (Topic 326). This ASU represents a significant change in the accounting for credit losses model by requiring immediate recognition of management’s estimates of current expected credit losses (CECL). Under the prior model, losses were recognized only as they were incurred. The Company has determined that it has met the criteria of a smaller reporting company (“SRC”) as of November 15, 2019. As such, ASU amended the effective date for the Company to be for reporting periods beginning after December 15, 2022. The Company will adopt this ASU effective July 1, 2023.
No. 2016-13:
Financial Instruments—Credit Losses
2019-10:
Financial Instruments-Credit Losses, Derivatives and Hedging, and Leases: Effective Dates
3. Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. For the nine months ended March 31, 2022, and 2021, the Company incurred a net loss of $13.1 million and $9.9 million and used net cash of $11.0 million and $7.7 million in operations, respectively. The Company expects to continue to incur additional operating losses in the foreseeable future.
12
The Company’s business focuses on the development of novel genetic medicines and, at this stage in the Company’s development, the Company has not established a source of revenue to cover its full operating costs, and as such, is dependent on funding operations through capital financing activitiesAs of March 31, 2022, the Company had $8.6 million in cash and cash equivalents. The Company does not have adequate liquidity to fund its operations for the next 12 months without raising additional funds and the success of raising such additional capital is not solely within the control of the Company. These factors raise substantial doubt about its ability to continue as a going concern.
.
The financial statements do not include any adjustments that might result from the outcome of this condition. If the Company is unable to raise additional funds, or the Company’s anticipated operating results are not achieved, management believes planned expenditures may need to be reduced or delayed in order to extend the time period that existing resources can fund the Company’s operations. The Company intends to fund ongoing activities by utilizing its current cash on hand and by raising additional capital. If the Company is unable to obtain the necessary capital, it may have a material adverse effect on the operations of the Company and the development of its technology (including delaying certain development milestones), or the Company may have to cease operations altogether
.
4. Prepaid and other assets
(US$’000) |
March 31, 2022 |
June 30, 2021 |
||||||
Prepaid expenses |
$ | $ | ||||||
Security deposit |
||||||||
Market value of listed shares |
||||||||
Total other assets |
||||||||
Less: non-current portion |
( |
) | ( |
) | ||||
Current portion |
$ | $ | ||||||
5. Property and equipment, net
(US$’000) |
March 31, 2022 |
June 30, 2021 |
||||||
Software |
$ | $ | ||||||
Lab equipment |
||||||||
Computer hardware |
||||||||
Leasehold improvements |
||||||||
Total property and equipment, gross |
||||||||
Accumulated depreciation and amortization |
( |
) | ( |
) | ||||
Total property and equipment, net |
$ | $ | ||||||
Depreciation expense was $54 thousand and $161 thousand for the three and nine months ended March 31, 2022, and $67 thousand and $179 thousand, respectively, for the same periods in 2021.
6. Trade and other payables
(US$’000) |
March 31, 2022 |
June 30, 2021 |
||||||
Trade payable |
$ | $ | ||||||
Accrued license fees |
||||||||
Accrued professional fees |
||||||||
Accrued research and development |
||||||||
Other payables |
||||||||
Total |
$ | $ | ||||||
13
7. Leases
The Company has entered into an operating lease for office space under an agreement that expires in 2022. The lease requires the Company to pay utilities, insurance, taxes and other operating expenses. The Company’s lease does not contain any residual value guarantees or material restrictive covenants. During August 2021, the Company extended the lease through June 2025 .
The tables below show the changes during the nine months ended March 31,
2022
: (US$’000) |
Operating lease right-of-
use assets |
|||
Balance at July 1, 2021 |
$ | |||
Re-measurement during the period |
||||
Amortization of right of use asset |
( |
) | ||
Operating lease right-of-use |
$ | |||
(US$’000) |
Operating lease liabilities |
|||
Balance at July 1, 2021 |
$ | |||
Re-measurement during the period |
||||
Principal payments on operating lease liabilities |
( |
) | ||
Operating lease liabilities at March 31, 2022 |
||||
Less: non-current portion |
( |
) | ||
Current portion at March 31, 2022 |
$ | |||
As of March 31, 2022, the Company’s operating lease has a remaining lease term of 3.21 years and a discount rate of 4.67 %. The maturities of the operating lease liabilities are as follows:
(US$’000) |
March 31, 202 2
|
|||
2022 |
$ |
|||
2023 |
||||
2024 |
||||
2025 |
||||
Total operating lease payments |
||||
Less imputed interest |
( |
) | ||
Present value of operating lease liabilities |
$ | |||
The Company recorded lease liabilities and lease assets for the lease based on the present value of lease payments over the expected lease term, discounted using the Company’s incremental borrowing rate. Rent expense was $0.1 million and $0.2 million for the three and nine months ended March 31, 2022, respectively, and $0.1 million and $0.2 million, respectively, for the same periods in 2021.
right-of-use
8. Stockholders’ equity
Common Stock
On October 6, 2020, the Company announced the closing of an underwritten public offering of 2,666,644 shares of its common stock at a price to the public of $3.10 per share. The Company also announced that the underwriter fully exercised its over-allotment option to purchase 483,870 additional shares of its common stock at the offering price of $3.10 per share. The gross and net proceeds were $11.5 million and $9.9 million, respectively.
On April 30, 2021, the Company announced the closing of an underwritten public offering of 3,036,366 shares of its common stock at a price to the public of $4.25 per share. The Company also announced that the underwriter exercised the over-allotment option to purchase 317,274 additional shares of its common stock at the offering price of $4.25 per share. The gross and net proceeds were $14.3 million and $12.7 million, respectively.
14
On December 8, 2021, the stockholders of the Company approved an amendment (the “Charter Amendment”) to the Company’s Amended and Restated Certificate of Incorporation to increase the total number of authorized shares of common stock of the Company from 10,000,000 to 40,000,000 . The Charter Amendment was filed with the Secretary of State of the State of Delaware and became effective on December 17, 2021.
Warrants
On December 6, 2019, the Investors were issued 4 Purchase Warrants that were exercisable into 214,190 fully paid shares of common stock should the Purchase Warrants be exercised in full (“Purchase Warrants”). The exercise price for the Purchase Warrants is US$10.50 per share issued on exercise of a Purchase Warrant. The Purchase Warrants are exercisable, in whole or in part, any time from the date of issue until the anniversary of the date of issue (December 6, 2024 ). On April 22, 2020, the Company issued 37,417 shares of common stock in connection with a cashless exercise of Purchase Warrants exercisable for 107,095 shares of common stock. The Company did not have an effective registration statement registering the resale of the Warrant Shares by the Holder at the time the Holder wanted to exercise the warrant, therefore, the Holder carried out a cashless exercise. The formula for conducting a cashless exercise was outlined in the Warrant agreement. Based on this formula, the Holder would have been entitled to receive 107,095 shares of common stock if they had exercised the Purchase Warrants for cash. Because of the cashless exercise, the holder received 37,417 shares. As of March 31, 2022, there were 107,095 of the warrants still outstanding.
On October 6, 2020, the Company announced the closing of an underwritten public offering of 559,162 shares of common stock underlying 3.09 per share and immediately exercisable at $0.01 per share 559,162
pre-funded
warrants initially purchased for $(“Pre-Funded
Warrants”). All Pre-Funded
Warrants issued had been exercised as of June 30, 2021. The activity related to warrants during for the
nine
months ended March 31, 2022, is summarized as follows: | Common Stock from Warrants |
Weighted- average Exercise Price (per share) |
|||||||
| Outstanding at July 1, 2021 |
$ | |||||||
| |
|
|
|
|||||
| Outstanding and exercisable at March 31, 2022 |
$ | |||||||
Equity Incentive Plan
Employee Share Option Plan
In connection with its
re-domiciliation
to the United States, the Company assumed BBL’s obligations with respect to the settlement of options that were issued by BBL prior to the re-domiciliation
pursuant to the Benitec Officers’ and Employees’ Share Option Plan (the “Share Option Plan”). This includes the Company’s assumption of the Share Option Plan and all award agreements pursuant to which each of the options were granted. Each option when exercised entitles the option holder to one share in the Company. Options are exercisable on or before an expiry date, do not carry any voting or dividend rights and are not transferable except on death of the option holder or in certain other limited circumstances. Employee options vest one third on each anniversary of the applicable grant date for three years. If an employee dies, retires or otherwise leaves the Company and certain exercise conditions have been satisfied, generally, the employee has 12 months to exercise their options or the options are cancelled. Since the re-domiciliation,
no new options have been or will be issued under the Share Option Plan. Equity and Incentive Compensation Plan
On December 9, 2020, the Company’s stockholders approved the Company’s 2020 Equity and Incentive Compensation Plan and, on December 8, 2021, the Company’s stockholders approved an amendment to increase the maximum number of shares that may be issued under such plan to 1,850,000 (as amended, the “2020 Plan”). The 2020 Plan provides for the grant of various equity awards. Currently, only stock options are issued under the 2020 Plan. Each option when exercised entitles the option holder to one share of the Company’s common stock. Options are exercisable on or before an expiry date, do not carry any voting or dividend rights, and are not transferable except on death of the option holder or in certain other limited circumstances. Employee stock options vest in increments of
one-third
on each anniversary of the applicable grant date for three years. Non-employee
director options vest in increments of one-third
on the day prior to each of the Company’s next three annual stockholder meetings following the grant date. If an option holder dies or terminates employment or service due to Disability (as defined in the 2020 Plan) and certain exercise conditions have been satisfied, generally, the option holder has 12 months to exercise their options or the options are cancelled. If an option holder 15
otherwise leaves the Company, other than for a termination by the Company for Cause (as defined in the 2020 Plan) and certain exercise conditions have been satisfied, generally, the option holder has 90 days to exercise their options or the options are cancelled. Future equity grants will be made under the 2020 Plan.
Equity Awards
The activity related to equity awards, which are comprised of stock options during the nine months ended March 31, 2022 is summarized as follows:
| Stock Options |
Weighted- average Exercise Price |
Weighted- average Remaining Contractual Term |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at July 1, 2021 |
$ |
|
$ | — | ||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at September 30, 2021 |
|
$ | — | |||||||||||||
| Granted |
|
$ | — | |||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Outstanding at March 31, 2022 |
|
$ | — | |||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Exercisable at March 31, 2022 |
$ |
|
$ | — | ||||||||||||
| |
|
|
|
|||||||||||||
Share-Based Compensation Expense
The classification of share-based compensation expense is summarized as follows:
Three Months Ended March 31, |
Nine Months Ended March 31, |
|||||||||||||||
(US$’000) |
2022 |
2021 |
2022 |
2021 |
||||||||||||
| Research and development |
$ | $ | $ | $ | ||||||||||||
| General and administrative |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total share-based compensation expense |
$ | $ | $ | $ | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
As of March 31, 2022, there was $0.6 million of unrecognized share-based compensation expense related to stock options issued under the Share Option Plan and the 2020 Plan.
9. Income taxes
For the three and nine months ended March 31, 2022, and 2021, the Company did not recognize a provision or benefit for income taxes as it has incurred net losses. In addition, the net deferred tax assets generated from net operating losses are fully offset by a valuation allowance as the Company believes it is more likely than not that the benefit will not be realized.
10. Commitments and contingencies
Contract commitments
The Company enters into contracts in the normal course of business with third-party contract research organizations, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancellable contracts and not considered contractual obligations and commitments.
Contingencies
From time to time, the Company may become subject to claims and litigation arising in the ordinary course of business. The Company is not a party to any material legal proceedings, nor is it aware of any material pending or threatened litigation.
11. Related party transactions
During the nine months ended March 31, 2022, the Company had entered into a related party transaction with Francis Abourizk Lightowlers for legal fees totaling $1 thousand. Peter Francis, a 0 and $2 thousand, respectively, included in trade and other payables on the accompanying consolidated balance sheet.
non-executive
director of the Company is a partner at Francis Abourizk Lightowlers. As of March 31, 2022 and June 30, 2021 there were amounts due to this related party of $16
12. Subsequent events
The Company has evaluated subsequent events through the filing of this Quarterly Report on Form
10-Q,
and determined that there have been no events that have occurred that would require adjustments or disclosures in the consolidated financial statements. Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of financial condition and operating results together with our consolidated financial statements and the related notes and other financial information included elsewhere in this document.
Overview
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders.
Benitec Biopharma Inc. (“Benitec” or the “Company” or in the third person, “we” or “our”) is a development-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary platform, called
DNA-directed
RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The Company is developing a ddRNAi-based therapeutic (BB-301)
for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), a chronic, life-threatening genetic disorder. BB-301
is a ddRNAi-based genetic medicine currently under development by Benitec. BB-301
is an AAV-based
gene therapy designed to simultaneously silence the expression of the mutant, disease-causing gene (to slow, or halt, the biological mechanisms underlying disease progression in OPMD) and replace the mutant gene with a wild type gene (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called “silence and replace”. The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301
has been granted Orphan Drug Designation in the United States and the European Union. The targeted gene silencing effects of RNAi, in conjunction with the durable transgene expression achievable via the use of modified viral vectors, imbues the silence and replace approach with the potential to produce long-term silencing of disease-causing genes along with simultaneous replacement of wild type gene function following a single administration of the proprietary genetic medicine. We believe that this novel mechanistic profile of the current and future investigational agents developed by Benitec could facilitate the achievement of robust and durable clinical activity while greatly reducing the frequency of drug administration traditionally expected for medicines employed for the management of chronic diseases. Additionally, the achievement of long-term gene silencing and gene replacement may significantly reduce the risk of patient
non-compliance
during the course of medical management of potentially fatal clinical disorders. COVID-19
COVID-19
has been declared a pandemic by the World Health Organization and has spread to nearly every country, including Australia and the United States. The impact of this pandemic has been, and will likely continue to be, extensive in many aspects of society, which has resulted in, and will likely continue to result in, significant disruptions to businesses and capital markets around the world. The extent to which the coronavirus impacts us will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the coronavirus and its variants, and the actions to contain the coronavirus or treat its impact, including the effectiveness and adoption of vaccines for the virus, among others. Certain elements of our research and development efforts are conducted globally, including the ongoing development of our silence and replace therapeutic for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), and will be dependent upon our ability to initiate preclinical and clinical studies despite the ongoing
COVID-19
pandemic. As we continue to actively advance our development programs, including our ongoing Toxicology and Biodistribution study for
BB-301,
we are in close contact with our principal investigators and preclinical trial sites, which are primarily located in France, and are assessing the impact of COVID-19
on our studies and the expected development timelines and costs on an ongoing basis. In light of developments relating to the COVID-19
global pandemic since the beginning of the outbreak, the focus of healthcare providers and hospitals on fighting the virus, and consistent with the FDA’s industry guidance for conducting clinical trials, we have experienced delays to the original timeline regarding the initiation and anticipated completion of the ongoing BB-301
Clinical Trial Application (CTA)-enabling and Investigational New Drug Application (IND)-enabling development work. The initiation of the BB-301
Pilot Dosing Study in Beagle dogs, which represents a key component of the CTA-enabling
and IND-enabling
work, was delayed by several months, however, the study has been completed without incident. The acquisition of chemical reagents, biological reagents and laboratory supplies which are essential for the conduct of basic laboratory research, the conduct of nonclinical studies and the 17
completion of GMP manufacturing of
BB-301,
has also become challenging due to the disruption of global supply chains inherent to the production of these materials. We will continue to evaluate the impact of the COVID-19
pandemic on our business and we expect to reevaluate the timing of our anticipated preclinical and clinical milestones as we learn more and the impact of COVID-19
on our industry becomes clearer. We have also implemented a halt of
non-essential
business travel and a rotation system whereby staff work from home and attend the laboratory on designated days which may result in a reduction of laboratory work. As we transition our employees back to our premises, there is a risk that COVID-19
infections occur at our offices or laboratory facilities and significantly affect our operations. Additionally, if any of our critical vendors are impacted, our business could be affected if we become unable to procure essential equipment in a timely manner or obtain supplies or services in adequate quantities and at acceptable prices. Development Programs
Our Pipeline
The following table sets forth the current product candidate and the development status:
Table 1. Pipeline: Oculopharyngeal Muscular Dystrophy

BB-301
We are developing
BB-301
for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), and BB-301
is currently undergoing evaluation in CTA-enabling
and IND-enabling
studies. BB-301
is the lead investigational agent under development by Benitec, and the key attributes of OPMD and BB-301
are outlined in Figure 3. Figure 3. Overview of the
BB-301
Program 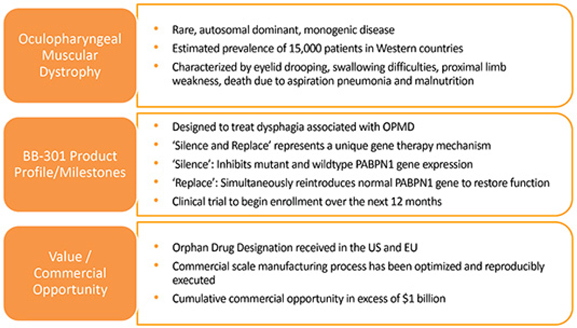
BB-301
is a first-in-class
40-to-50
18
OPMD is a rare disease, however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder. Each of these attributes could facilitate efficient clinical development and global
ommercialization of c
BB-301.
PABPN1 is a ubiquitous factor that promotes the interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage. The characteristic genetic mutation underlying OPMD results in trinucleotide repeat expansion(s) within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the
N-terminal
end of PABPN1. The mutation generates a protein with an N-terminal
expanded poly-alanine tract of up to 18 contiguous alanine residues, and the mutant protein is prone to the formation of intranuclear aggregates designated as intranuclear inclusions (INIs). The INIs that sequester wildtype PABPN1 may contribute to the “loss of function” phenotype associated with OPMD. No therapeutic agents are approved for the treatment of OPMD. Additionally, there are no surgical interventions available to OPMD patients that modify the natural history of the disease, which is principally comprised of chronic deterioration of swallowing function.
BB-301
has received Orphan Drug Designation in the United States and the European Union and, upon achievement of regulatory approval for BB-301
in these respective jurisdictions, the Orphan Drug Designations would provide commercial exclusivity independent of intellectual property protection. While OPMD is a rare medical disorder, we believe the commercial opportunity for a safe and efficacious therapeutic agent in this clinical indication exceeds $1 billion over the course of the commercial life of the product. Benitec has previously outlined the core
CTA-enabling
and IND-enabling
studies required by global regulatory agencies to support the initiation of BB-301
clinical trials in OPMD patients, and these studies include a BB-301
Pilot Dosing Study (the “Pilot Dosing Study”) in large animals and a classical 12-week
GLP Toxicology and Biodistribution Study for BB-301.
In these large animal studies, BB-301
is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality which characterizes the natural history of OPMD in human subjects. As referenced above, the
BB-301
Pilot Dosing Study in large animals was the first of two CTA-enabling
and IND-enabling
studies conducted by Benitec. This study was carried out under the guidance of the scientific team at Benitec, with key elements of the design and execution of the study conducted in close collaboration with a team of experts in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for decades. The BB-301
Pilot Dosing Study and the GLP Toxicology and Biodistribution Study for BB-301
were conducted in canine subjects in order to: | • | Support the validation and optimization of the newly designed route and method of BB-301 administration, |
| • | Confirm the efficiency of vector transduction and transgene expression in the key tissue compartments underlying the morbidity and mortality that comprises the natural history of OPMD, |
| • | Confirm the optimal BB-301 doses in advance of initiation of human clinical studies, |
| • | Facilitate the observation of key toxicological data-points. |
The
BB-301
Pilot Dosing Study was designed as an 8-week
study in Beagle dogs to confirm the transduction efficiency of BB-301
upon administration via direct intramuscular injection into specific anatomical regions of the pharynx through the use of an open surgical procedure. This new method and route of BB-301
administration was developed in collaboration with key surgical experts in the field of Otolaryngology, and this novel method of BB-301
dosing will significantly enhance the ability of treating physicians to accurately administer the AAV-based
investigational agent to the muscles that underlie the characteristic deficits associated with disease progression in OPMD. It is important to note that prior BB-301
non-clinical
studies have reproducibly validated the robust biological activity achieved following direct intramuscular injection of the AAV-based
agent. As an example, direct injection of BB-301
into the tibialis anterior muscles of A17 mice facilitated robust transduction of the targeted skeletal muscle cells and supported complete remission of the OPMD disease phenotype in this animal model. Benitec conducted the
BB-301
Pilot Dosing Study in Beagle dog subjects to demonstrate that direct intramuscular injection of BB-301
via the use of a proprietary dosing device in an open surgical procedure could safely achieve the following goals: | • | Biologically significant and dose-dependent levels of BB-301 tissue transduction (i.e., delivery of the multi-functional BB-301 genetic construct into the target pharyngeal muscle cells), |
| • | Broad-based and dose-dependent expression of the three distinct genes comprising the BB-301 gene construct within the pharyngeal muscle cells, and |
| • | Biologically significant levels of target gene knock-down (i.e., inhibition of the expression of the gene of interest) within the pharyngeal muscle cells. |
The Pilot Dosing Study evaluated the safety and biological activity of two concentrations of
BB-301
(1.0+E13 vg/mL and 3.0+E13 vg/mL) across three distinct doses (1.0+E13 vg/mL and 3.0+E13 vg/mL with a low injection volume, and 3.0+E13 19
vg/mL with a high injection volume) following direct intramuscular injection into the Hypopharyngeus (HP) muscles and the Thyropharyngeus (TP) muscles of Beagle dogs via the use of a proprietary delivery device employed in an open surgical procedure. The HP muscle in Beagle dogs corresponds to the Middle Pharyngeal Constrictor muscle in human subjects, and the TP muscle in Beagle dogs corresponds to the Inferior Pharyngeal Constrictor muscle in human subjects.
BB-301
was injected only on Day 1 of the Pilot Dosing Study, and the corresponding canine pharyngeal muscles were harvested for molecular analyses after 8 weeks of observation post-injection. BB-301
dosing was carried out independently by a veterinary surgeon and an Otolaryngologist with extensive experience regarding the provision of palliative surgical care for OPMD patients. Molecular analyses have been completed for the canine subjects treated in the
BB-301
Pilot Dosing Study. Key interim data-sets derived from the analyses of pharyngeal muscle tissues isolated from 16 Beagle dog subjects (of the 24-subject
Beagle dog study population) are highlighted below. The final data-set
derived from the completed molecular analyses of the pharyngeal muscle tissues of the canine subjects treated on the Pilot Dosing Study will be presented in a peer-reviewed format. The key interim data-sets are summarized below:
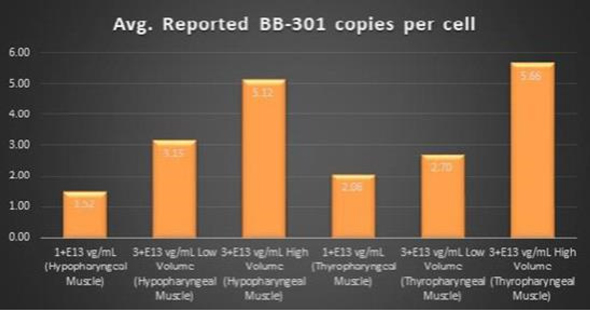
Figure 4. Pharyngeal Muscle Tissue Transduction Levels Achieved by
BB-301
Regarding Gene Expression Levels Observed for
BB-301
Within the Pharyngeal Muscle Tissues (Figure 5, Figure 6, Figure 7): | • | BB-301 encodes two distinct siRNA species (i.e., siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e., “silencing”) the expression of the mutant form of the PABPN1 protein and the wild type (i.e., endogenous) form of the PABPN1 protein (importantly, the mutant form of the PABPN1 protein underlies the development, and progression, of OPMD). |
| • | BB-301 also codes for a wild type version of the PABPN1 protein whose intracellular expression is unaffected by the inhibitory activities of siRNA13 and siRNA17; this “codon optimized” transcript drives the expression of a PABPN1 protein (i.e., coPABPN1) which serves to replenish the endogenous form of the PABPN1 protein and to replace the mutant form of PABPN1 that underlies the development and progression of OPMD in diseased tissues. |
| • | For comparative purposes, it should be noted that the average range of expression for wild type PABPN1 within the pharyngeal muscle cells of Beagle dogs is 4.5 copies per cell-to-7.8 |
20
Figure 5. siRNA13 Expression Levels Achieved by
BB-301
within Pharyngeal Muscle Tissues 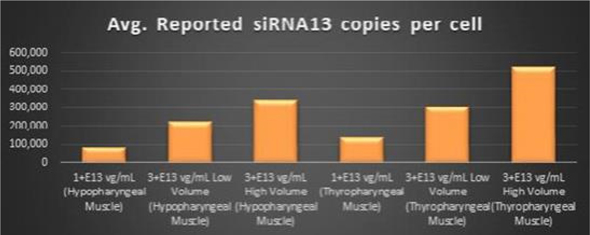
Figure 6. siRNA17 Expression Levels Achieved by
BB-301
within Pharyngeal Muscle Tissues 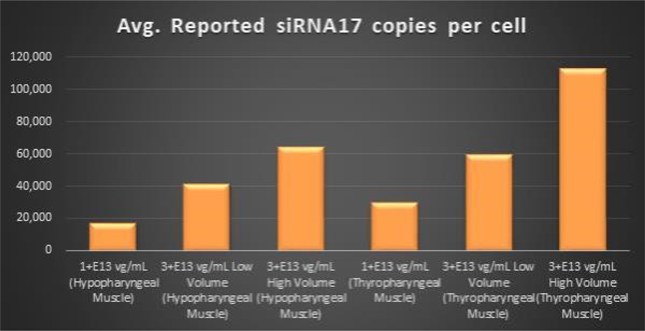
21
Figure 7. coPABPN1 Expression Levels Achieved by
BB-301
within Pharyngeal Muscle Tissues 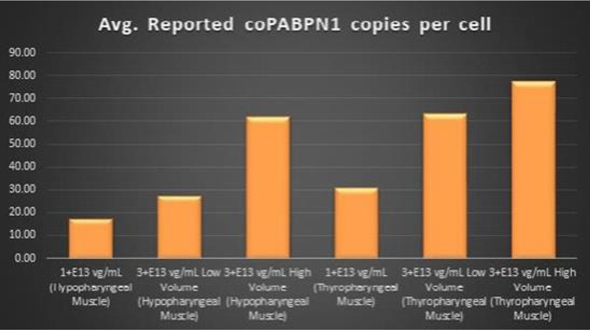
Regarding Wild Type PABPN1 Silencing (i.e., target “knock-down”) Observed for
BB-301
Within the Pharyngeal Muscle Tissues (Figure 8): | • | As noted above, BB-301 encodes two distinct siRNA species (i.e., siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e., “silencing”) the expression of all forms of the PABPN1 protein (siRNA13 and siRNA17 silence the expression of both wild type PABPN1 [wtPABPN1] and mutant PABPN1). |
| • | While the Beagle dog subjects treated in the BB-301 Pilot Dosing Study do not express mutant PABPN1, the level of BB-301-driven |
| • | Thus, the wtPABPN1 silencing activity observed in the BB-301 Pilot Dosing Study serves as a surrogate for the silencing activity that would be anticipated in the presence of mutant PABPN1. |
| • | BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and, as a result, manifest the symptomatic phenotype of OPMD; in the symptomatic animal model of OPMD (i.e. the A17 mouse model), the achievement of PABPN1 silencing levels of 31% inhibition (or higher) following BB-301 administration led to resolution of OPMD disease symptoms and the elimination of the histopathological hallmarks of OPMD. |
22
Figure 8. PABPN1 Silencing (i.e., “target knock-down”) Achieved by
BB-301
within Pharyngeal Muscle Tissues 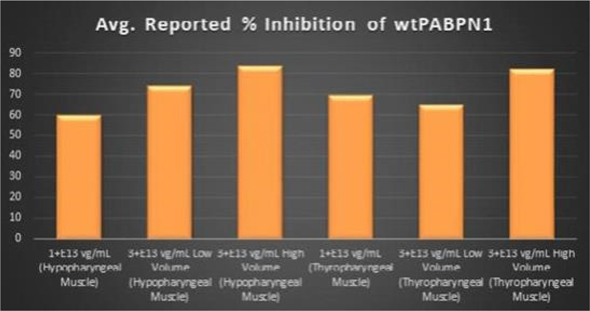
There are key methodological distinctions between the current
BB-301
Pilot Dosing Study conducted by Benitec as compared to the prior BB-301
Beagle dog dosing study carried out independently by the previous BB-301
licensee. The BB-301
dosing study conducted by the prior BB-301
licensee employed non-ideal
routes and methods of BB-301
administration to the target pharyngeal muscle tissues and employed similarly limited analytical methods at the completion of the dosing phase of the study. Subsequently, the Benitec team worked to optimize the route and method of administration of BB-301
and to refine the core analytical methods employed following the completion of dosing of the large animal subjects. The current proprietary method of
BB-301
delivery to the key pharyngeal muscles of study subjects, and the proprietary molecular analytical methods employed to assay the pharyngeal muscle tissues of study subjects, with both methods having been developed by the Benitec team, led to the observation of broad-based transduction of the targeted pharyngeal muscle tissues (Figure 9, represents individual sections of the TP muscle following BB-301
dosing). Critically, the Benitec-developed methods also facilitated the achievement of a 228-fold
improvement (+22,647%) in BB-301
transduction of the HP muscle and a 113-fold
improvement (+11,163%) in BB-301
transduction of the TP muscle relative to the levels of BB-301
transduction observed by the previous BB-301
licensee at identical BB-301
doses in identical canine study populations (Figure 10). Figure 9.
BB-301
Transduction Levels Achieved for Individual Sections of the TP Muscle Following BB-301
dosing 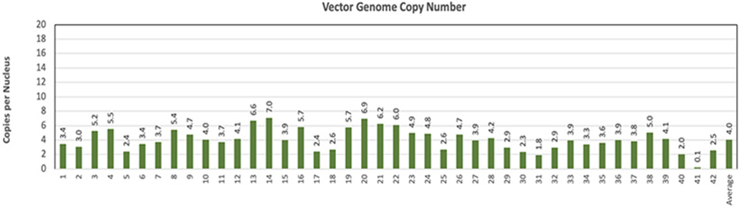
23
Figure 10. Impact of the Methodological Improvements to the
BB-301
Large Animal Dosing Study Design on the Relative Pharyngeal Muscle Tissue Transduction Levels Achieved by Benitec vs. the Former BB-301
Licensee 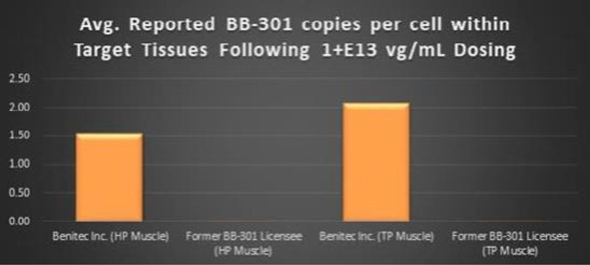
Following the disclosure of the positive interim
BB-301
Pilot Dosing Study results, Benitec completed pre-CTA
and pre-IND
meetings with regulatory agencies in France, Canada, and the United States. Summary
of Regulatory Interactions:
| • | Benitec successfully completed the regulatory interactions required to support initiation of the BB-301 clinical development program in 2022 |
| • | Successful regulatory engagement comprised the completion of the following meetings: |
| • | Pre-Clinical Trial Application (Pre-CTA) Consultation Meeting with Health Canada |
| • | Scientific Advice Meeting with The National Agency for the Safety of Medicines and Health Products in France (L’Agence nationale de sécurité du médicament et des produits de santé or “ANSM”) |
| • | Type C Meeting with the U.S. Food and Drug Administration (“FDA”) |
Benitec will begin the clinical development program for
BB-301
in 2022. Summary of the
BB-301
Clinical Development Program: | • | The BB-301 clinical development program will begin in 2022, and the conduct of the development program will comprise approximately 76-weeks of follow-up for each OPMD study participant, inclusive of: |
| • | 6-month pre-treatment observation periods employing quantitative radiographic imaging techniques for evaluation of the baseline disposition and natural history of OPMD-derived dysphagia in each study participant |
24
| • | 1 day of BB-301 dosing to initiate participation in the Phase 1b/2a single-arm, open-label, sequential, dose escalation cohort study |
| • | 52-weeks of post-dosing follow-up for conclusive evaluation of the primary and secondary endpoints of the Phase 1b/2a BB-301 treatment study |
| • | The OPMD Natural History Study will begin in 2022, and this observational study will facilitate the characterization of OPMD patient disposition at baseline and assess subsequent rates of progression of dysphagia (swallowing impairment) in subjects with OPMD via the use of quantitative radiographic measures of global swallowing function and pharyngeal constrictor muscle function along with clinical assessments and patient-reported self-assessments of swallowing function |
| • | Videofluoroscopic Swallowing Studies (VFSS) will be conducted to complete the following methodological assessments: |
| • | Dynamic Imaging Grade of Swallowing Toxicity Scale (DIGEST) |
| • | Pharyngeal Area at Maximum Constriction (PhAMPC) |
| • | Pharyngeal Constriction Ratio (PCR) |
| • | Clinical measures of global swallowing capacity and oropharyngeal dysphagia |
| • | Patient-reported measures of oropharyngeal dysphagia |
| • | The natural history of dysphagia observed for each OPMD study participant, as characterized by the quantitative radiographic measures and the clinical and patient self-reported assessments outlined above, will serve as the baseline for comparative assessments of safety and efficacy of BB-301 upon rollover of OPMD study subjects from the Natural History Study onto the Phase 1b/2a BB-301 treatment study |
25
| • | Upon the achievement of 6-months of follow-up in the Natural History Study, OPMD Natural History Study participants can become eligible for enrollment onto the Phase 1b/2a treatment study with the investigational genetic medicine, BB-301, which uses an AAV9-based gene therapy approach for the treatment of OPMD-derived dysphagia |
| • | This first-in-human BB-301 administered to the pharyngeal muscles of subjects with OPMD |
| • | Upon rollover from the Natural History Study onto the Phase 1b/2a BB-301 treatment study, the follow-up of OPMD study participants will continue for 52-weeks, and the primary endpoints (safety and tolerability) and secondary endpoints (comprising the quantitative radiographic measures of global swallowing function and pharyngeal constrictor muscle function, and the clinical and patient-reported assessments noted above) will be evaluated during each 90-day period following Day 1 (Day 1 represents the day of BB-301 intramuscular injection). |
Royalties, milestone payments and other license fees
We are required to pay royalties, milestone payments and other license fees in connection with our licensing of intellectual property from third parties, including as discussed below.
We have collaborated with Biomics Biotechnologies Co., Ltd., or Biomics, pursuant to several collaboration agreements in relation to single-stranded RNA and shRNA sequences for treatment of hepatitis B. In July 2015, we entered into an
earn-out
agreement with Biomics which confirmed Benitec’s ownership of certain patents resulting from the collaboration in exchange for an upfront payment and equity issuance to Biomics and a share of certain future licensing revenue received by Benitec. Foreign Currency Translation and Other Comprehensive Income (Loss)
The Company’s functional currency and reporting currency is the United States dollar. BBL’s functional currency is the Australian dollar (AUD). Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Equity transactions are translated at each historical transaction date spot rate. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive loss.” Gains and losses resulting from foreign currency translation are included in the consolidated statements of operations and comprehensive loss as other comprehensive income (loss).
Other comprehensive income for all periods presented includes only foreign currency translation gains.
October 2020 Capital Raise
On October 6, 2020, the Company announced the closing of an underwritten public offering of common stock and common stock equivalents. The Company received gross proceeds of approximately $11.5 million and net proceeds of approximately $9.9 million from the offering.
April 2021 Capital Raise
On April 30, 2021, the Company announced the closing of an underwritten public offering of common stock and common stock equivalents. The Company received gross proceeds of approximately $14.3 million and net proceeds of approximately $12.7 million from the offering.
Results of Operations
Revenues
The Company has not generated any revenues from the sales of products. Revenues from licensing fees are included in the revenue from customers line item on our consolidated statements of operations and comprehensive loss. Our licensing fees have been
26
generated through the licensing of our ddRNAi technology to biopharmaceutical companies. The following table sets forth a summary of our revenues for each of the periods set forth below:
| Three Months Ended March 31, |
Nine Months Ended March 31, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
(US$’000) |
||||||||||||||||
| Revenues: |
||||||||||||||||
| Licensing revenues from customers |
$ | 48 | $ | 1 | $ | 73 | $ | 57 | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total revenues |
$ | 48 | $ | 1 | $ | 73 | $ | 57 | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
Revenues from customers
During the nine months ended March 31, 2022 and 2021, respectively, the Company recognized $73 thousand and $57 thousand in customer revenues. For the three months ended March 31, 2022 and 2021, respectively, the Company recognized $48 thousand and $1 thousand in customer revenues. The increase in revenues from customers is due to the increase in licensing revenues in the current period.
Royalties and License Fees
Royalties and license fees consist primarily of payments we are required to remit for royalties and other payments related to
in-licensed
intellectual property. Under our in-license
agreements, we may pay up-front
fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. As our product sales increase, we may, from time to time, disagree with our third-party collaborators as to the appropriate royalties owed, and the resolution of such disputes may be costly, may consume management’s time, and may damage our relationship with our collaborators. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments. Research and Development Expenses
Research and development expenses relate primarily to the cost of conducting clinical and
pre-clinical
trials. Pre-clinical
and clinical development costs are a significant component of research and development expenses. The Company records accrued liabilities for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of pre-clinical
studies and clinical trials, and contract manufacturing activities. The Company records the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and includes these costs in trade and other payables on the consolidated balance sheets and within research and development expenses on the consolidated statements of operations and comprehensive loss. The Company accrues for these costs based on factors such as estimates of the work completed and in accordance with agreements established with its third-party service providers. The Company makes significant judgments and estimates in determining the accrued liabilities balance at the end of each reporting period. As actual costs become known, the Company adjusts its accrued liabilities. The Company has not experienced any material differences between accrued costs and actual costs incurred.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, related benefits, travel, and equity-based compensation expense. General and administrative expenses also include facility expenses, professional fees for legal, consulting, accounting and audit services and other related costs.
We anticipate that our general and administrative expenses may increase as the Company focuses on the continued development of the
pre-clinical
OPMD program. The Company also anticipates an increase in expenses relating to accounting, legal and regulatory-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and other similar costs. 27
Operating Expenses
The following tables sets forth a summary of our expenses for each of the periods:
| Three Months Ended March 31, |
Nine Months Ended March 31, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
(US$’000) |
||||||||||||||||
| Operating Expenses: |
||||||||||||||||
| Royalties and license fees |
$ | — | $ | 7 | $ | — | $ | 122 | ||||||||
| Research and development |
2,171 | 2,758 | 8,096 | 4,700 | ||||||||||||
| General and administrative |
1,337 | 1,029 | 5,093 | 4,976 | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
$ | 3,508 | $ | 3,794 | $ | 13,189 | $ | 9,798 | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
During the three and nine months ended March 31, 2022, respectively, we incurred $0 in royalties and license fees, as compared to $7 thousand and $122 thousand, respectively, for the comparable periods ended March 31, 2021.
During the three and nine months ended March 31, 2022, respectively, we incurred $2.2 million and $8.1 million in research and development expenses, as compared to $2.8 million and $4.7 million, respectively, for the comparable periods ended March 31, 2021. The decrease in research and development expenses for the three-month period relates primarily to the
BB-301
GMP-grade
BB-301,
BB-301
. The increase for the nine-month period also relates to the
BB-301
non-clinical
B-301
General and administrative expense was $1.3 million and $5.1 million for the three and nine months ended March 31, 2022, as compared to $1.0 million and $5.0 million for the comparable periods ended March 31, 2021. The increase for the three and nine month periods was due to increases in insurance, consultants, legal and accounting fees, and share-based compensation.
28
Other Income (Expense)
The following tables sets forth a summary of our other income (loss) for each of the periods:
| Three Months Ended March 31, |
Nine Months Ended March 31, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
(US$’000) |
||||||||||||||||
| Other Income (Loss): |
||||||||||||||||
| Foreign currency transaction gain (loss) |
$ | 229 | $ | (113 | ) | $ | 36 | $ | (167 | ) | ||||||
| Interest expense, net |
(10 | ) | (2 | ) | (22 | ) | (5 | ) | ||||||||
| Other income (expense), net |
(29 | ) | 1 | (29 | ) | 37 | ||||||||||
| Unrealized loss on investment |
(5 | ) | (2 | ) | (10 | ) | (3 | ) | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total other income (loss), net |
$ | 185 | $ | (116 | ) | $ | (25 | ) | $ | (138 | ) | |||||
| |
|
|
|
|
|
|
|
|||||||||
The other income (loss), net during the three and nine months ended March 31, 2022, respectively, totaled $185 thousand and $(25) thousand, which consists of foreign currency transaction gains, interest expense, other income, and unrealized gain on investment. During the three and nine months ended March 31, 2021, respectively, other income (expense), net totaled $(116) thousand and $(138) thousand. Foreign currency transaction losses have swung to gains due to a change in foreign exchange rates. Other income, net decreased due to no longer receiving
COVID-19
stimulus incentives from the Australian government in 2022. Unrealized loss on investment increased for the three and nine months ended March 31, 2022, compared to the three and nine months ended March 31, 2021. Liquidity and Capital Resources
The Company has incurred cumulative losses and negative cash flows from operations since our predecessor’s inception in 1995. The Company had accumulated losses of $143 million as of March 31, 2022. We expect that our research and development expenses may increase due to the continued development of the OPMD program. It is also likely that there will be an increase in the general and administrative expenses due to the obligations of being a domestic public company in the United States.
We had no borrowings as of March 31, 2022 and do not currently have a credit facility.
As of March 31, 2022, we had cash and cash equivalents of approximately $8.6 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts.
The following table sets forth a summary of the net cash flow activity for each of the periods set forth below:
| Nine Months Ended March 31, |
||||||||
2022 |
2021 |
|||||||
(US$’000) |
||||||||
| Net cash provided by (used in): |
||||||||
| Operating activities |
$ | (11,044 | ) | $ | (7,675 | ) | ||
| Investing activities |
— | (362 | ) | |||||
| Financing activities |
— | 9,854 | ||||||
| Effects of exchange rate changes on cash and cash equivalents |
(95 | ) | 370 | |||||
| |
|
|
|
|||||
| Net (decrease) increase in cash |
$ | (11,139 | ) | $ | 2,187 | |||
| |
|
|
|
|||||
Operating activities
Net cash used in operating activities for the nine months ended March 31, 2022 and 2021 was $11.0 million and $7.7 million, respectively. Net cash used in operating activities was primarily the result of our net loss, partially offset by
non-cash
expenses, and changes in working capital, including an increase in payables. Investing activities
Net cash used in investing activities for the nine months ended March 31, 2022 and 2021 was $0 and $0.4 million, respectively. The change was primarily related to a decrease in purchases of equipment in 2022 as there were none compared to purchases of $0.4 million in the same period of 2021.
29
Financing activities
Net cash provided by financing activities was $0 and $9.9 million for the nine months ended March 31, 2022 and 2021, respectively. Cash from financing activities in 2021 was related to the issuance of common stock, including $11.5 million in gross proceeds from the October 2020 Capital Raise, partially offset by $1.6 million in share issuance costs.
The future of the Company as an operating business will depend on its ability to manage operating costs and budgeted amounts and obtain adequate financing. While we continue to progress discussions and advance opportunities to engage with pharmaceutical companies and continue to seek licensing partners for ddRNAi in disease areas that are not our focus, there can be no assurance as to whether we will enter into such arrangements or what the terms of any such arrangement could be.
While we have established some licensing arrangements, we do not have any products approved for sale and have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates.
Unless and until we establish significant revenues from licensing programs, strategic alliances or collaboration arrangements with pharmaceutical companies, or from product sales, we anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of product candidates and begin to prepare to commercialize any product that receives regulatory approval. We are subject to the risks inherent in the development of new gene therapy products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The Company does not have adequate liquidity to fund its operations for the next 12 months without raising additional funds and the success of raising such additional capital is not solely within the control of the Company. These factors raise substantial doubt about its ability to continue as a going concern. See Note 3.
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| • | the timing and costs of our planned clinical trials for our ddRNAi and silence and replace product candidates; |
| • | the timing and costs of our planned preclinical studies for our ddRNAi and silence and replace product candidates; |
| • | the number and characteristics of product candidates that we pursue; |
| • | the outcome, timing and costs of seeking regulatory approvals; |
| • | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| • | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may establish; |
| • | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| • | the extent to which we need to in-license or acquire other products and technologies. |
Contractual Obligations and Commercial Commitments
On October 1, 2016, the Company entered into an operating lease for office space in Hayward, California that originally expired in April 2018. The Company has entered into lease amendments that extended the lease through June 2025.
The Company enters into contracts in the normal course of business with third-party contract research organizations, contract development and manufacturing organizations and other service providers and vendors. These contracts generally provide for termination on notice and, therefore, are cancellable contracts and not considered contractual obligations and commitments.
Critical Accounting Policies and Significant Accounting Estimates
The preparation of consolidated financial statements and related disclosures in conformity with accounting principles generally accepted in the United States of America requires management to make judgments, assumptions and estimates that affect the amounts reported. Note 2 of the Notes to Consolidated Financial Statements included in this Quarterly Report on Form
10-Q
describes the significant accounting policies used in the preparation of the consolidated financial statements. Certain of these significant accounting policies are considered to be critical accounting policies. 30
A critical accounting policy is defined as one that is both material to the presentation of the Company’s consolidated financial statements and requires management to make difficult, subjective or complex judgments that could have a material effect on the Company’s financial condition or results of operations. Specifically, these policies have the following attributes: (1) the Company is required to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates the Company could reasonably have used, or changes in the estimate that are reasonably likely to occur, would have a material effect on the Company’s financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. The Company bases its estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained and as the Company’s operating environment changes. These changes have historically been minor and have been included in the consolidated financial statements as soon as they became known. In addition, management is periodically faced with uncertainties, the outcomes of which are not within its control and will not be known for prolonged periods of time. These uncertainties are discussed in the section above entitled “Risk Factors.” Based on a critical assessment of its accounting policies and the underlying judgments and uncertainties affecting the application of those policies, management believes that the Company’s consolidated financial statements are fairly stated in accordance with accounting principles generally accepted in the United States of America and provide a meaningful presentation of the Company’s financial condition and results of operations.
Management believes that the following are critical accounting policies:
Research and Development Expense
Research and development expenses relate primarily to the cost of conducting clinical and
pre-clinical
trials. Pre-clinical
and clinical development costs are a significant component of research and development expenses. The Company records accrued liabilities for estimated costs of research and development activities conducted by third-party service providers, which include the conduct of pre-clinical
studies and clinical trials, and contract manufacturing activities. The Company records the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and includes these costs in trade and other payables on the consolidated balance sheets and within research and development expenses on the consolidated statements of operations and comprehensive loss. The Company accrues for these costs based on factors such as estimates of the work completed and in accordance with agreements established with its third-party service providers. The Company makes significant judgments and estimates in determining the accrued liabilities balance at the end of each reporting period. As actual costs become known, the Company adjusts its accrued liabilities. The Company has not experienced any material differences between accrued costs and actual costs incurred.
Share-based Compensation Expense
The Company records share-based compensation in accordance with ASC 718,. ASC 718 requires the fair value of all share-based employee compensation awarded to employees and
Stock Compensation
non-employees
to be recorded as an expense over the shorter of the service period or the vesting period. The Company determines employee and non-employee
share-based compensation based on grant-date fair value using the Black-Scholes Option Pricing Model. Recent Accounting Pronouncements
Accounting Standards recently adopted
None.
New Accounting Standards and Interpretations not yet mandatory or early adopted
ASU
2016-13
No. 2016-13:
“Financial Instruments-Credit Losses
2019-10:
“Financial Instruments-Credit Losses, Derivatives and Hedging, and Leases: Effective Dates”
31
Item 3. Quantitative and Qualitative Disclosures About Market Risk
As a smaller reporting company, we are not required to provide the information pursuant to this Item.
Item 4. Controls and Procedures
We have established disclosure controls and procedures (as defined in Rules
13a-15(e)
and 15d-15(e)
under the Securities Exchange Act of 1934, as amended). As of the end of the period covered by this Report we carried out an evaluation under the supervision and with the participation of our management, including our principal executive officer and principal financial and accounting officer, of the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15
of the Securities and Exchange Act of 1934, as amended. Based upon that evaluation, our principal executive officer and principal financial and accounting officer concluded that our disclosure controls and procedures are effective. There were no changes in our internal controls over financial reporting during the quarter ended March 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
We do not expect that our disclosure controls and procedures or our internal controls will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected.
32
PART II
OTHER INFORMATION
Item 1. Legal Proceedings
We are currently not a party to any material legal proceedings.
Item 1A. Risk Factors
There have been no material changes to the risk factors disclosed in Item 1A of the Company’s Annual Report on Form
10-K
for the fiscal year ended June 30, 2021. Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
None.
Item 5. Other Information
None.
Item 6. Exhibits.
| Number | Description of Document | |
| 31.1 | ||
| 31.2 | ||
| 32.1 | ||
| 32.2 | ||
| 101.INS | Inline XBRL Instance Document* | |
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document* | |
| 101.CAL | Inline XBRL Calculation Linkbase Document* | |
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document* | |
| 101.LAB | Inline XBRL Label Linkbase Document* | |
| 101.PRE | Inline XBRL Taxonomy Presentation Linkbase Document* | |
| 104 | Cover Page Interactive Data File—the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |
| * | Filed herewith. |
| ** | Furnished, not filed. |
33
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on our behalf by the undersigned thereunto duly authorized.
| Benitec Biopharma Inc. | ||
| Dated: May 16, 2022 |
||
| /s/ Jerel Banks | ||
| Jerel Banks | ||
| Executive Chairman and Chief Executive Officer (principal executive officer) | ||
| /s/ Megan Boston | ||
| Megan Boston | ||
| Executive Director (principal financial and accounting officer) | ||
34
