S-1/A: General form of registration statement for all companies including face-amount certificate companies
Published on September 9, 2022
Table of Contents
As filed with the Securities and Exchange Commission on September 8, 2022
Registration No. 333-266417
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
To
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Benitec Biopharma Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 84-462-0206 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
3940 Trust Way
Hayward, California 94545
(510) 780-0819
(Address, including zip code, and telephone number, including area code, of registrants principal executive offices)
Dr. Jerel Banks
Chief Executive Officer
3940 Trust Way
Hayward, California 94545
(510) 780-0819
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Matthew S. OLoughlin, Esq. Ben D. Orlanski, Esq. Louis Rambo, Esq. Proskauer Rose LLP 2029 Century Park East, Suite 2400 Los Angeles, CA 90067-3010 (310) 557-2900 (310) 557-2193-Facsimile |
Barry I. Grossman, Esq. Sarah Williams, Esq. Matthew Bernstein, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, NY 10105 (212) 370-1300 (212) 370-7889-Facsimile |
Approximate date of commencement of proposed sale to public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of large accelerated filer, accelerated filer, smaller reporting company and emerging growth company in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 8, 2022
Preliminary Prospectus

$20,000,000
25,380,710 Shares of Common Stock or
Pre-funded Warrants to Purchase 25,380,710 Shares of Common Stock and
Common Warrants to Purchase 25,380,710 Shares of Common Stock
Shares of Common Stock underlying the Pre-funded Warrants and Series 1 Common Warrants
We are offering 25,380,710 shares of our common stock together with common warrants to purchase shares of our common stock (and the shares of common stock that are issuable from time to time upon exercise of the Series 1 Common Warrants (as defined below)). The common warrants will be issued separately but must be purchased together with the common stock and/or the pre-funded warrants (as described below). The combined purchase price for each share of common stock and accompanying common warrant is $ and for each pre-funded warrant and accompanying common warrant is $ . The common warrants will be exercisable beginning on the date of issuance (the Series 1 Common Warrants) or, as applicable (as further described herein), the date on which we (a) receive approval from our stockholders (the Stockholder Approval) to increase the number of shares of common stock we are authorized to issue (a Capital Event) and (b) effect such Capital Event by filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation (each such date, an Initial Exercise Date) (the Series 2 Common Warrants), at an exercise price of $ per share and will expire on the five-year anniversary of the Initial Exercise Date.
We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, pre-funded warrants in lieu of shares of common stock that would otherwise result in any such purchasers beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock and will be exercisable at any time after its original issuance until exercised in full. The purchase price of each pre-funded warrant will be equal to the price at which a share of common stock are sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. This offering also relates to the shares of common stock issuable upon exercise of the Series 1 Common Warrants or any pre-funded warrants sold in this offering.
Our common stock is listed on The Nasdaq Capital Market under the symbol BNTC. On September 8, 2022, the last reported sale price of our common stock on The Nasdaq Capital Market was $0.7880. There is no established public trading market for the pre-funded warrants or common warrants, and we do not expect a market to develop. We do not intend to apply for listing of the pre-funded warrants or common warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants or common warrants will be limited.
The public offering price per share of common stock and/or any pre-funded warrant, together with the common warrant that accompanies common stock or a pre-funded warrant will be determined between us, the underwriter and purchasers based on market conditions at the time of pricing, and may be at a discount to the current market
Table of Contents
price. Therefore, the recent market price used throughout this prospectus may not be indicative of the actual public offering price for our common stock, our pre-funded warrants and the common warrants. There is no established public trading market for the pre-funded warrants or common warrants, and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the pre-funded warrants or common warrants on any national securities exchange. Without an active trading market, the liquidity of the common warrants and the pre-funded warrants will be limited.
You should read this prospectus, together with additional information described under the heading Where You Can Find More Information, carefully before you invest in any of our securities.
Investing in our securities involves a high degree of risk. See Risk Factors beginning on page 28.
|
Per Share of Common Stock and Common Warrant(1) |
Per Pre-Funded Warrant and Common Warrant |
Total(3) | ||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions(2) |
$ | $ | $ | |||||||||
| Proceeds to us (before expenses) |
$ | $ | $ | |||||||||
| (1) | Based on an assumed public offering price of $ per share of common stock and common warrant. The final public offering price per share of common stock or pre-funded warrant, together with the common warrant that accompanies common stock or a pre-funded warrant, as the case may be, will be determined by us, the underwriter and the purchasers in this offering and may be a discount to the current market price of our common stock. |
| (2) | We have agreed to reimburse certain expenses of the underwriter which are not included in the table above. See Underwriting for a description of the compensation payable to the underwriter. |
| (3) | Assumes no pre-funded warrants are issued in lieu of shares of common stock. |
We have granted the underwriter a 30-day option to purchase an aggregate of up to 3,807,106 additional shares of our common stock and/or up to 3,807,106 additional common warrants to purchase 3,807,106 shares of our common stock from us at the public offering price per share of common stock and common warrant, less the underwriting discounts and commissions. The underwriter may exercise its option to acquire additional shares for the sole purpose of covering over-allotments. See Underwriting.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Delivery of the securities offered hereby is expected to be made on or about , 2022.
Sole Book-Running Manager
JMP Securities
A CITIZENS COMPANY
The date of this prospectus is , 2022
Table of Contents
| Page | ||||
| 1 | ||||
| 2 | ||||
| 2 | ||||
| 3 | ||||
| 5 | ||||
| 23 | ||||
| 26 | ||||
| 28 | ||||
| 34 | ||||
| 35 | ||||
| 36 | ||||
| Market Price of Our Common Stock and Related Stockholder Matters |
38 | |||
| 38 | ||||
| 39 | ||||
| 41 | ||||
| 48 | ||||
| 57 | ||||
| 62 | ||||
| 62 | ||||
| 63 | ||||
| 63 | ||||
i
Table of Contents
This prospectus is part of a registration statement on Form S-1 that we filed with the Securities and Exchange Commission (SEC). You should rely only on the information contained in this prospectus or contained in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not, and the underwriter has not, authorized anyone to provide you with information that is different from that contained in such prospectuses. We are offering to sell securities and are seeking offers to buy securities only in jurisdictions where such offers and sales are permitted. For investors outside the United States: We have not, and the underwriter has not, taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby and the distribution of this prospectus outside the United States. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our securities.
This prospectus and the information incorporated herein by reference contain summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled Where You Can Find Additional Information. We urge you to read carefully this prospectus, together with the information incorporated herein by reference before deciding whether to participate in the offering hereunder.
We further note that the representations, warranties and covenants made by us in any document that is filed as an exhibit to the registration statement of which this prospectus is a part and in any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Unless the context otherwise requires, the terms Benitec, the Company, we, us, our and similar terms used in this prospectus refer (i), prior to the Re-domiciliation (as defined herein) to Benitec Biopharma Limited (BBL), an Australian corporation, and its subsidiaries, and (ii), following the Re-domiciliation, to Benitec Biopharma Inc., a Delaware corporation, and its subsidiaries (including Benitec Limited). Any references to Benitec Limited or BBL refer to Benitec Biopharma Limited, an Australian corporation. On August 14, 2020, BBL reorganized as a Proprietary Limited company and changed its name to Benitec Biopharma Proprietary Limited.
All references to $ in this prospectus refer to U.S. dollars. All references to A$ in this prospectus mean Australian dollars. As of June 30, 2022, the rate of exchange of U.S. dollars to Australian dollars was 1.44839 AUD.
Our fiscal year-end is June 30. References to a particular fiscal year are to our fiscal year ended June 30 of that calendar year.
1
Table of Contents
This prospectus includes information with respect to market and industry conditions and market share from third-party sources or based upon estimates using such sources when available. We believe that such information and estimates are reasonable and reliable. We also believe the information extracted from publications of third-party sources has been accurately reproduced. However, we have not independently verified any of the data from third-party sources. Similarly, our internal research is based upon our understanding of industry conditions, and such information has not been verified by any independent sources.
We have proprietary and licensed rights to trademarks used in this prospectus which are important to our business, many of which are registered under applicable intellectual property laws. These trademarks include:
| | BENITEC BIOPHARMA® |
| | BENITEC® |
| | GIVING DISEASE THE SILENT TREATMENT® |
| | SILENCING GENES FOR LIFE® |
Solely for convenience, trademarks and trade names referred to in this prospectus appear without the ® or symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name or service mark of any other company appearing in this prospectus is the property of its respective holder.
2
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. Our forward-looking statements relate to future events or our future performance and include, but are not limited to, statements concerning our business strategy, future commercial revenues, market growth, capital requirements, new product introductions, expansion plans and the adequacy of our funding. All statements, other than statements of historical fact included in this prospectus, are forward-looking statements. When used in this prospectus, the words could, believe, anticipate, intend, estimate, expect, may, continue, predict, potential, project, or the negative of these terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements.
Some of the risks and uncertainties that may cause our actual results, performance or achievements to differ materially from those expressed or implied by forward-looking statements include the following:
| | the success of our plans to develop and potentially commercialize our product candidates; |
| | the timing of the initiation and completion of preclinical studies and clinical trials; |
| | the timing and sufficiency of patient enrollment and dosing in any future clinical trials; |
| | the timing of the availability of data from clinical trials; |
| | the timing and outcome of regulatory filings and approvals; |
| | unanticipated delays; |
| | sales, marketing, manufacturing and distribution requirements; |
| | market competition and the acceptance of our products in the marketplace; |
| | regulatory developments in the United States, France and Canada; |
| | the development of novel AAV (as defined below) vectors; |
| | the plans of licensees of our technology; |
| | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a one shot cure; |
| | our dependence on our relationships with collaborators and other third parties; |
| | expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors, including our capital structure; |
| | our ability to continue as a going concern; |
| | the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; |
| | our intellectual property position and the duration of our patent portfolio; |
| | the impact of local, regional, and national and international economic conditions and events; |
| | the impact of the current COVID-19 pandemic, the disease caused by the SARS-CoV-2 virus, which may adversely impact our business and preclinical and future clinical trials; and |
| | our ability to receive Stockholder Approval and effect the Capital Event. |
as well as other risks detailed under the caption Risk Factors in this prospectus and in other reports filed with the SEC. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and important factors
3
Table of Contents
currently known by us and our expectations of the future, about which we cannot be certain. We have based the forward-looking statements included in this prospectus and in the documents incorporated herein by reference on information available to us on the date of this prospectus or on the date thereof. Except as required by law we undertake no obligation to revise or update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised to consult any additional disclosures that we may make directly to you or through reports that we, in the future, may file with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K.
All forward-looking statements included herein or in documents incorporated herein by reference are expressly qualified in their entirety by the cautionary statements contained or referred to elsewhere in this prospectus.
4
Table of Contents
This summary highlights information contained in other parts of this prospectus and in the documents incorporated by reference herein and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus and the documents incorporated by reference herein, including our consolidated financial statements and the related notes, and the information set forth under the section titled Risk Factors, as well as those risk factors included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 and any subsequent Quarterly Report on Form 10-Q. Some of the statements in this prospectus and the documents incorporated by reference herein constitute forward-looking statements that involve risks and uncertainties. See the information set forth under the section Special Note Regarding Forward-Looking Statements.
Company Overview
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders.
Benitec Biopharma Inc. (Benitec or the Company or in the third person, we or our) is a development-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary platform, called DNA-directed RNA interference, or ddRNAi, combines RNA interference, or RNAi, with gene therapy to create medicines that facilitate sustained silencing of disease-causing genes following a single administration. The Company is developing a ddRNAi-based therapeutic (BB-301) for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), a chronic, life-threatening genetic disorder.
BB-301 is a ddRNAi-based genetic medicine currently under development by Benitec. BB-301 is an AAV-based gene therapy designed to simultaneously silence the expression of the mutant, disease-causing gene (to slow, or halt, the biological mechanisms underlying disease progression in OPMD) and replace the mutant gene with a wild type gene (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called silence and replace. The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
The targeted gene silencing effects of RNAi, in conjunction with the durable transgene expression achievable via the use of modified viral vectors, imbues the silence and replace approach with the potential to produce long-term silencing of disease-causing genes along with simultaneous replacement of wild type gene function following a single administration of the proprietary genetic medicine. We believe that this novel mechanistic profile of the current and future investigational agents developed by Benitec could facilitate the achievement of robust and durable clinical activity while greatly reducing the frequency of drug administration traditionally expected for medicines employed for the management of chronic diseases. Additionally, the achievement of long-term gene silencing and gene replacement may significantly reduce the risk of patient non-compliance during the course of medical management of potentially fatal clinical disorders.
We will require additional financing to progress our product candidates through to key inflection points.
5
Table of Contents
Recent Developments
On September 6, 2022, we received a letter from the Listing Qualifications Department of the Nasdaq Stock Market notifying us that the minimum bid price per share for our common stock fell below $1.00 for a period of 30 consecutive business days and that therefore we did not meet the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2).
The letter also states that we will be provided 180 calendar days, or until March 6, 2023, to regain compliance with the minimum bid price requirement. In accordance with Rule 5810(c)(3)(A), we can regain compliance if at any time during the 180-day period the closing bid price of our common stock is at least $1.00 for a minimum of 10 consecutive business days. If by January 6, 2020, we cannot demonstrate compliance with the Rule 5550(a)(2), we may be eligible for additional time. To qualify for additional time, we will be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the bid price requirement, and we will need to provide written notice of our intention to cure the deficiency during the second compliance period. If we are not eligible for the second compliance period, then the Nasdaq Staff will provide notice that our securities will be subject to delisting. At such time, we may appeal the delisting determination to a Hearings Panel.
We intend to monitor the closing bid price of our common stock and may, if appropriate, consider implementing available options to regain compliance with the minimum bid price requirement. These options include completing a reverse stock split of our common stock for the purpose of meeting the closing bid price requirement. We have previously completed reverse stock splits in connection with complying with our listing requirement and any such option we undertake will not, in of itself, cause us to remain in compliance.
Our Strengths
We believe that the combination of our proprietary ddRNAi technology and our deep expertise in the design and development of genetic medicines, and specifically ddRNAi-based therapeutics, will enable us to achieve and maintain a leading position in gene silencing and gene therapy for the treatment of human disease. Our key strengths include:
| | A first mover advantage for ddRNAi-based therapeutics; |
| | A proprietary ddRNAi-based silence and replace technology platform that may potentially enable the serial development of single-administration therapeutics capable of facilitating sustained, long-term silencing of disease-causing genes and concomitant replacement of wild type gene function; |
| | A proprietary AAV vector technology which improves the endosomal escape capability of virus produced in insect cells using a baculovirus system. This technology has broad application in AAV-based gene therapies; |
| | The capabilities to drive the development of a pipeline of programs focused on chronic diseases with either large patient populations, or rare diseases, which may potentially support the receipt of Orphan Drug Designation, including OPMD; and |
| | A growing portfolio of patents protecting improvements to our ddRNAi, and silence and replace, technology and product candidates through at least 2036, with additional patent life anticipated through at least 2040. |
Our Strategy
We endeavor to become the leader in discovery, development, and commercialization of therapeutic agents capable of addressing significant unmet medical need via the application of the silence and replace approach to the treatment of genetic disorders. We apply the following general strategy to drive the Company towards these goals:
| | Selectively develop proprietary and partnered programs; and |
6
Table of Contents
| | Continue to explore and secure research and development partnerships with global biopharmaceutical companies supported by the differentiated nature of our scientific platform and intellectual property portfolio. |
Our senior leadership team will continue to explore partnership opportunities with global biopharmaceutical companies, as we expect that the unique attributes of the proprietary ddRNAi and silence and replace approaches, and the breadth of potential clinical indications amenable to our proprietary methods, to support the formation of collaborations over a broad range of diseases with significant unmet medical need.
We seek to actively protect our intellectual property and proprietary technology. These efforts are central to the growth of our business and include:
| | Seeking and maintaining patents claiming our ddRNAi and silence and replace technologies and other inventions relating to our specific products in development or that are otherwise commercially and/or strategically important to the development of our business; |
| | Protecting and enforcing our intellectual property rights; and |
| | Strategically licensing intellectual property from third parties to advance development of our product candidates. |
Our TechnologyddRNAi and Silence and Replace
Our proprietary technology platforms are designated as DNA-directed RNA interference, or ddRNAi, and silence and replace. ddRNAi is designed to produce long-term silencing of disease-causing genes, by combining RNA interference, or RNAi, with viral delivery agents typically associated with the field of gene therapy (i.e., viral vectors). Modified AAV vectors are employed to deliver genetic constructs which encode short hairpin RNAs that are, then, serially expressed and processed to produce siRNA molecules within the transduced cell for the duration of the life of the target cell. These newly introduced siRNA molecules drive long-term, and potentially permanent, silencing of the expression of the disease-causing gene. The silence and replace approach further bolsters the biological benefits of long-term silencing of disease-causing genes by incorporating multifunctional genetic constructs within the modified AAV vectors to create an AAV-based gene therapy agent that is designed to both silence the expression of mutated, disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and, simultaneously, replace the mutant genes with normal, wild type genes (to drive restoration of function in diseased cells). This fundamentally distinct therapeutic approach to disease management offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of diseases like Oculopharyngeal Muscular Dystrophy (OPMD).
Traditional gene therapy is defined by the introduction of an engineered transgene to correct the pathophysiological derangements derived from mutated or malfunctioning genes. Mutated genes can facilitate the intracellular production of disease-causing proteins or hamper the production of critical, life-sustaining, proteins. The introduction of a new transgene can facilitate the restoration of production of normal proteins within the diseased cell, thus, restoring natural biological function. Critically, the implementation of this traditional method of gene therapy cannot eliminate the expression, or the potential deleterious effects of, the underlying mutant gene (as mutant proteins may be continually expressed and aggregate or drive the aggregation of other native proteins within the diseased cell). In this regard, the dual capabilities of the proprietary silence and replace approach to silence a disease-causing gene via ddRNAi and simultaneously replace the wild type activity of a mutant gene via the delivery of an engineered transgene could facilitate the development of differentially efficacious treatments for a range of genetic disorders.
7
Table of Contents
Overview of RNAi and the siRNA Approach
The mutation of a single gene can cause a chronic disease via the resulting intracellular production of a disease-causing protein (i.e. an abnormal form of the protein of interest), and many chronic and/or fatal disorders are known to result from the inappropriate expression of a single gene or multiple genes. In some cases, genetic disorders of this type can be treated exclusively by silencing the intracellular production of the disease-causing protein through well-validated biological approaches like RNA interference (RNAi). RNAi employs small nucleic acid molecules to activate an intracellular enzyme complex, and this biological pathway temporarily reduces the production of the disease-causing protein. In the absence of the disease-causing protein, normal cellular function is restored and the chronic disease that initially resulted from the presence of the mutant protein is partially or completely resolved. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes.
Figure 1. The siRNA Approach
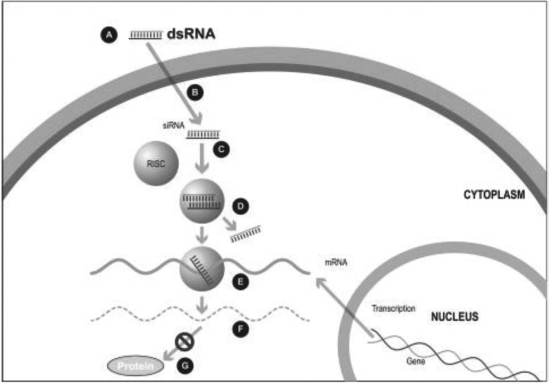
A small double stranded RNA, or dsRNA, molecule (A, Figure 1), comprising one strand known as the sense strand and another strand known as the antisense strand, which are complementary to each other, is synthesized in the laboratory. These small dsRNAs are called small interfering RNAs, or siRNAs. The sequence of the sense strand corresponds to a short region of the target gene mRNA. The siRNA is delivered to the target cell (B, Figure 1), where a group of enzymes, referred to as the RNA-Induced Silencing Complex, or RISC, process the siRNA (C, Figure 1), where one of the strands (usually the sense strand) is released (D, Figure 1). RISC uses the antisense strand to find the mRNA that has a complementary sequence (E, Figure 1) leading to the cleavage of the target mRNA (F, Figure 1). As a consequence, the output of the mRNA (protein production) does not occur (G, Figure 1). Several companies, including Alnylam Pharmaceuticals Inc. (Alnylam) and Arbutus Biopharma Corp. (Arbutus), utilize this approach in their RNAi product candidates.
8
Table of Contents
Importantly, many genetic disorders are not amenable to the traditional gene silencing approach outlined in Figure 1, as the diseased cells may produce a mixture of the wild type protein of interest and the disease-causing mutant variant of the protein, and the underlying genetic mutation may be too small to allow for selective targeting of the disease-causing variant of the protein through the use of siRNA-based approaches exclusively. In these cases, it is extraordinarily difficult to selectively silence the disease-causing protein without simultaneously silencing the wild type intracellular protein of interest whose presence is vital to the conduct of normal cellular functions.
Our proprietary silence and replace technology utilizes the unique specificity and robust gene silencing capabilities of RNAi while overcoming many of the key limitations of siRNA-based approaches to disease management.
In the standard RNAi approach, double-stranded siRNA is produced synthetically and, subsequently, introduced into the target cell via chemical modification of the RNA or alternative methods of delivery. While efficacy has been demonstrated in several clinical indications through the use of this approach, siRNA-based approaches maintain a number of limitations, including:
| | Clinical management requires repeat administration of the siRNA-based therapeutic agent for multiple cycles to maintain efficacy; |
| | Long-term patient compliance challenges due to dosing frequencies and treatment durations; |
| | Therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the target cells decrease over time; |
| | Novel chemical modifications or novel delivery materials are typically required to introduce the siRNA into the target cells, making it complicated to develop a broad range of therapeutics agents; |
| | Potential adverse immune responses, resulting in serious adverse effects; |
| | Requirement for specialized delivery formulations for genetic disorders caused by mutations of multiple genes; and |
| | siRNA acts only to silence genes and cannot be used to replace defective genes with normally functioning genes. |
Our Approach to the Treatment of Genetic DiseasesddRNAi and Silence and Replace
Our proprietary silence and replace approach to the treatment of genetic diseases combines RNAi with wild type gene replacement to drive sustained silencing of disease-causing genes and concomitant restoration of functional wild type genes following a single administration of the therapeutic agent. Benitec employs ddRNAi in combination with classical gene therapy (i.e. transgene delivery via viral vectors) to overcome several of the fundamental limitations of RNAi.
The silence and replace approach to the treatment of genetic disorders employs adeno-associated viral vectors (AAVs) to deliver genetic constructs which may, after a single administration to the target tissues:
| | Chronically express RNAi molecules inside of the target, diseased, cells (to serially silence the intracellular production of mutant, disease-causing, protein and the wild type protein of interest); |
| | Simultaneously drive the expression of a wild type variant of the protein of interest (to restore native intracellular biological processes); and |
| | AAV vectors can accommodate the multi-functional DNA expression cassettes containing the engineered wild type transgenes and the novel genes encoding short hairpinRNA/microRNA molecules (shRNA/miRNA) that are required to support the development of therapeutic agents capable of the achievement of the goals of the silence and replace approach to therapy. |
9
Table of Contents
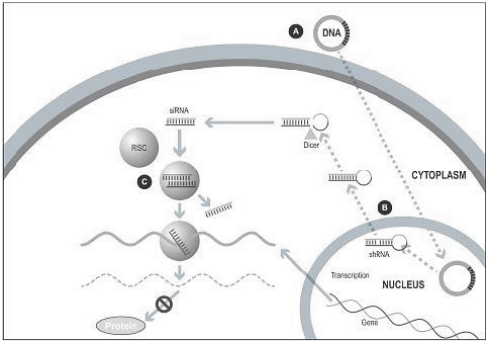
Our silence and replace technology utilizes proprietary DNA expression cassettes to foster continuous production of gene silencing shRNAs and wild type proteins (via expression of the wild type transgene). A range of viral and non-viral gene therapy vectors can be used to deliver the DNA construct into the nucleus of the target cell and, upon delivery, shRNA molecules are expressed and subsequently processed by intracellular enzymes into siRNA molecules that silence the expression of the mutant, disease-causing protein (Figure 2).
In the silence and replace approach (Figure 2):
| | A DNA construct is delivered to the nucleus of the target cell by a gene therapy vector (A) such as an AAV vector; |
| | Once inside of the nucleus, the DNA construct drives the continuous production of shRNA molecules (B) which are processed by an enzyme called Dicer into siRNAs (C); |
| | The processed siRNA is incorporated into RISC and silences the target gene using the same mechanism shown in Figure 1; and |
| | When the DNA expression cassette is additionally comprised of a wild type transgene, upon entry of the DNA construct into the nucleus of the target cell via the use of the AAV vector, the DNA construct also drives the continuous production of wild type protein (to restore native intracellular biological processes). |
Figure 2. The Silence and Replace Approach
Our strategy is to discover, develop and commercialize treatments that leverage the capabilities of ddRNAi and the silence and replace approach to disease management.
For selected product candidates, at the appropriate stage, we may collaborate with large biopharmaceutical companies to further co-develop and, if approved, commercialize our ddRNAi-based and silence and replace-based products to achieve broad clinical and commercial distribution. For specific clinical indications that we
10
Table of Contents
deem to be outside of our immediate areas of focus, we will continue to out-license, where appropriate, applications of our ddRNAi and silence and replace technology to facilitate the development of differentiated therapeutics, which could provide further validation of our proprietary technology and approach to disease management.
Our cash and cash equivalents will be deployed to advance our product candidate BB-301 for OPMD, including the natural history lead-in study and Phase 1b/2a BB 301 treatment study, for the continued advancement of development activities for other existing and new product candidates, for general corporate purposes and for strategic growth opportunities.
Oculopharyngeal Muscular DystrophyOPMD
OPMD is an insidious, autosomal-dominant, late-onset degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1, or PABPN1, gene. OPMD is a rare disease; however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder, which could simplify clinical development and global commercialization efforts.
BB-301 is an AAV-based gene therapy designed to both silence the expression of mutated, disease-causing genes (to slow, or halt, the underlying mechanism of disease progression) and simultaneously replace the mutant genes with normal, wild type genes (to drive restoration of function in diseased cells). This fundamental therapeutic approach to disease management is called silence and replace and this biological mechanism offers the potential to restore the underlying physiology of the treated tissues and, in the process, improve treatment outcomes for patients suffering from the chronic and, potentially, fatal effects of Oculopharyngeal Muscular Dystrophy (OPMD). BB-301 has been granted Orphan Drug Designation in the United States and the European Union.
On July 9, 2018, the Company entered into a License and Collaboration Agreement with Axovant. Pursuant to the Agreement, the Company granted Axovant an exclusive worldwide license to develop, manufacture, and commercialize products containing the Companys product known as BB-301, which was designed for the potential treatment of Oculopharyngeal Muscular Dystrophy. As of September 3, 2019, the License and Collaboration Agreement with Axovant was terminated. As a result, all rights and licenses which Benitec had granted to Axovant to develop and commercialize BB-301 and related gene therapy product candidates terminated. We are now solely responsible for the costs in connection with the development and commercialization of the BB-301 product candidates.
Prior to such termination, the Benitec team endeavored to conduct several additional exploratory nonclinical analyses in order to potentially improve the biological efficacy of BB-301 via further optimization of the route of administration employed to dose the target muscle tissues.
Our Pipeline
The following table sets forth the current product candidate and the development status:
Table 1. Pipeline: Oculopharyngeal Muscular Dystrophy

11
Table of Contents
BB-301
We are developing BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy (OPMD), and BB-301 is currently undergoing evaluation in CTA-enabling and IND-enabling studies. BB-301 is the lead investigational agent under development by Benitec, and the key attributes of OPMD and BB-301 are outlined in Figure 3.
Figure 3. Overview of the BB-301 Program
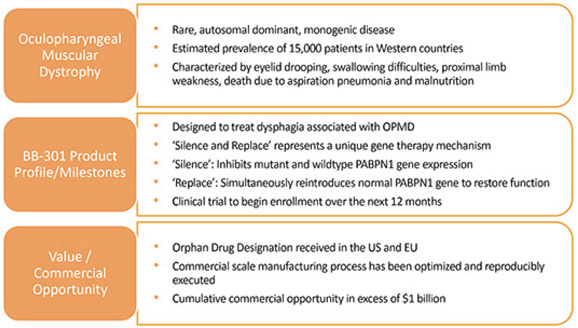
BB-301 is a first-in-class genetic medicine employing the silence and replace approach for the treatment of OPMD. OPMD is an insidious, autosomal-dominant, late-onset, degenerative muscle disorder that typically presents in patients at 40-to-50 years of age. The disease is characterized by progressive swallowing difficulties (dysphagia) and eyelid drooping (ptosis). OPMD is caused by a specific mutation in the poly(A)-binding protein nuclear 1 gene (PABPN1).
OPMD is a rare disease, however, patients have been diagnosed with OPMD in at least 33 countries. Patient populations suffering from OPMD are well-identified, and significant geographical clustering has been noted for patients with this disorder. Each of these attributes could facilitate efficient clinical development and global commercialization of BB-301.
PABPN1 is a ubiquitous factor that promotes the interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and, thus, controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage. The characteristic genetic mutation underlying OPMD results in trinucleotide repeat expansion(s) within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1. The mutation generates a protein with an N-terminal expanded poly-alanine tract of up to 18 contiguous alanine residues, and the mutant protein is prone to the formation of intranuclear aggregates designated as intranuclear inclusions (INIs). The INIs that sequester wild type PABPN1 may contribute to the loss of function phenotype associated with OPMD.
12
Table of Contents
No therapeutic agents are approved for the treatment of OPMD. Additionally, there are no surgical interventions available to OPMD patients that modify the natural history of the disease, which is principally comprised of chronic deterioration of swallowing function. BB-301 has received Orphan Drug Designation in the United States and the European Union and, upon achievement of regulatory approval for BB-301 in these respective jurisdictions, the Orphan Drug Designations would provide commercial exclusivity independent of intellectual property protection. While OPMD is a rare medical disorder, we believe the commercial opportunity for a safe and efficacious therapeutic agent in this clinical indication exceeds $1 billion over the course of the commercial life of the product.
Benitec has previously outlined the core CTA-enabling and IND-enabling studies required by global regulatory agencies to support the initiation of BB-301 clinical trials in OPMD patients, and these studies include a BB-301 Pilot Dosing Study (the Pilot Dosing Study) in large animals and a classical 12-week GLP Toxicology and Biodistribution Study for BB-301. In these large animal studies, BB-301 is directly injected into the pharyngeal muscles known to underlie the morbidity and mortality which characterizes the natural history of OPMD in human subjects.
As referenced above, the BB-301 Pilot Dosing Study in large animals was the first of two CTA-enabling and IND-enabling studies conducted by Benitec. This study was carried out under the guidance of the scientific team at Benitec, with key elements of the design and execution of the study conducted in close collaboration with a team of experts in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for decades. The BB-301 Pilot Dosing Study and the GLP Toxicology and Biodistribution Study for BB-301 were conducted in canine subjects in order to:
| | Support the validation and optimization of the newly designed route and method of BB-301 administration, |
| | Confirm the efficiency of vector transduction and transgene expression in the key tissue compartments underlying the morbidity and mortality that comprises the natural history of OPMD, |
| | Confirm the optimal BB-301 doses in advance of initiation of human clinical studies, and |
| | Facilitate the observation of key toxicological data-points. |
The BB-301 Pilot Dosing Study was designed as an 8-week study in Beagle dogs to confirm the transduction efficiency of BB-301 upon administration via direct intramuscular injection into specific anatomical regions of the pharynx through the use of an open surgical procedure. This new method and route of BB-301 administration was developed in collaboration with key surgical experts in the field of Otolaryngology, and this novel method of BB-301 dosing will significantly enhance the ability of treating physicians to accurately administer the AAV-based investigational agent to the muscles that underlie the characteristic deficits associated with disease progression in OPMD. It is important to note that prior BB-301 non-clinical studies have reproducibly validated the robust biological activity achieved following direct intramuscular injection of the AAV-based agent. As an example, direct injection of BB-301 into the tibialis anterior muscles of A17 mice facilitated robust transduction of the targeted skeletal muscle cells and supported complete remission of the OPMD disease phenotype in this animal model.
Benitec conducted the BB-301 Pilot Dosing Study in Beagle dog subjects to demonstrate that direct intramuscular injection of BB-301 via the use of a proprietary dosing device in an open surgical procedure could safely achieve the following goals:
| | Biologically significant and dose-dependent levels of BB-301 tissue transduction (i.e., delivery of the multi-functional BB-301 genetic construct into the target pharyngeal muscle cells), |
| | Broad-based and dose-dependent expression of the three distinct genes comprising the BB-301 gene construct within the pharyngeal muscle cells, and |
13
Table of Contents
| | Biologically significant levels of target gene knock-down (i.e., inhibition of the expression of the gene of interest) within the pharyngeal muscle cells. |
The Pilot Dosing Study evaluated the safety and biological activity of two concentrations of BB-301 (1.0+E13 vg/mL and 3.0+E13 vg/mL) across three distinct doses (1.0+E13 vg/mL and 3.0+E13 vg/mL with a low injection volume, and 3.0+E13 vg/mL with a high injection volume) following direct intramuscular injection into the Hypopharyngeus (HP) muscles and the Thyropharyngeus (TP) muscles of Beagle dogs via the use of a proprietary delivery device employed in an open surgical procedure. The HP muscle in Beagle dogs corresponds to the Middle Pharyngeal Constrictor muscle in human subjects, and the TP muscle in Beagle dogs corresponds to the Inferior Pharyngeal Constrictor muscle in human subjects. BB-301 was injected only on Day 1 of the Pilot Dosing Study, and the corresponding canine pharyngeal muscles were harvested for molecular analyses after 8 weeks of observation post-injection. BB-301 dosing was carried out independently by a veterinary surgeon and an Otolaryngologist with extensive experience regarding the provision of palliative surgical care for OPMD patients.
Molecular analyses have been completed for the canine subjects treated in the BB-301 Pilot Dosing Study. Key interim data-sets derived from the analyses of pharyngeal muscle tissues isolated from the Beagle dog subjects are highlighted below. The final data-set derived from the completed molecular analyses of the pharyngeal muscle tissues of the canine subjects treated on the Pilot Dosing Study will be presented in a peer-reviewed format.
The key interim data-sets are summarized below:
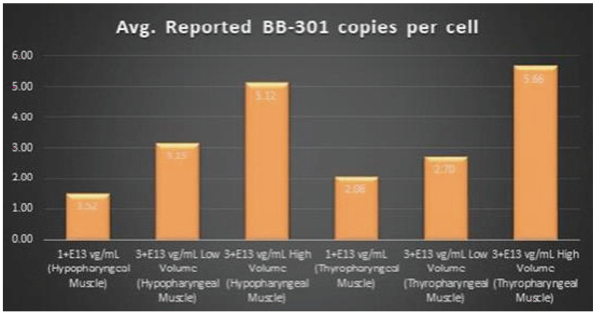
Figure 4. Pharyngeal Muscle Tissue Transduction Levels Achieved by BB-301
14
Table of Contents
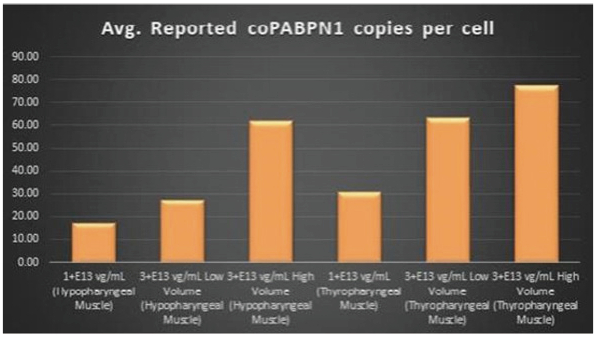
Regarding Gene Expression Levels Observed for BB-301 Within the Pharyngeal Muscle Tissues (Figure 5, Figure 6, Figure 7):
| | BB-301 encodes two distinct siRNA species (i.e., siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e., silencing) the expression of the mutant form of the PABPN1 protein and the wild type (i.e., endogenous) form of the PABPN1 protein (importantly, the mutant form of the PABPN1 protein underlies the development, and progression, of OPMD). |
| | BB-301 also codes for a wild type version of the PABPN1 protein whose intracellular expression is unaffected by the inhibitory activities of siRNA13 and siRNA17; this codon optimized transcript drives the expression of a PABPN1 protein (i.e., coPABPN1) which serves to replenish the endogenous form of the PABPN1 protein and to replace the mutant form of PABPN1 that underlies the development and progression of OPMD in diseased tissues. |
| | For comparative purposes, it should be noted that the average range of expression for wild type PABPN1 within the pharyngeal muscle cells of Beagle dogs is 4.5 copies per cell-to-7.8 copies per cell. |
Figure 5. siRNA13 Expression Levels Achieved by BB-301 within Pharyngeal Muscle Tissues
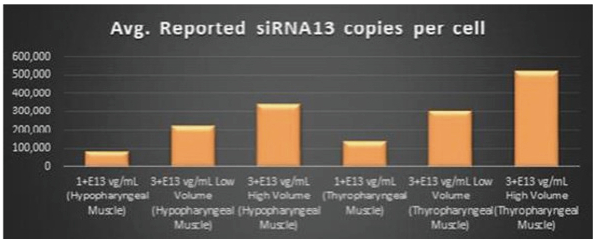
15
Table of Contents
Figure 6. siRNA17 Expression Levels Achieved by BB-301 within Pharyngeal Muscle Tissues
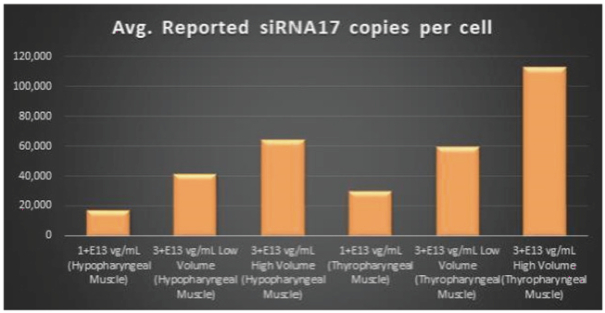
Figure 7. coPABPN1 Expression Levels Achieved by BB-301 within Pharyngeal Muscle Tissues
16
Table of Contents
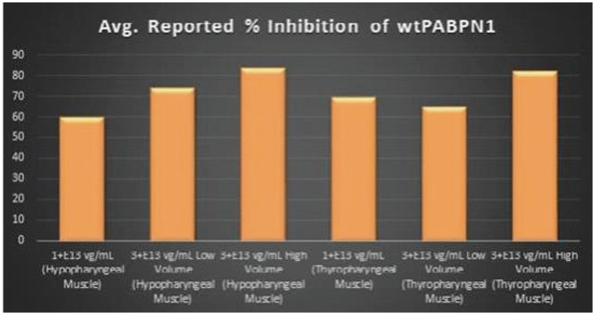
Regarding Wild Type PABPN1 Silencing (i.e., target knock-down) Observed for BB-301 Within the Pharyngeal Muscle Tissues (Figure 8):
| | As noted above, BB-301 encodes two distinct siRNA species (i.e., siRNA13 and siRNA17) which are each, independently, capable of inhibiting (i.e., silencing) the expression of all forms of the PABPN1 protein (siRNA13 and siRNA17 silence the expression of both wild type PABPN1 (wtPABPN1) and mutant PABPN1). |
| | While the Beagle dog subjects treated in the BB-301 Pilot Dosing Study do not express mutant PABPN1, the level of BB-301-driven gene silencing for the PABPN1 target can be indirectly assessed in these study subjects due to the equivalent inhibitory effects of siRNA13 and siRNA17 on both wtPABPN1 and mutant PABPN1. |
| | Thus, the wtPABPN1 silencing activity observed in the BB-301 Pilot Dosing Study serves as a surrogate for the silencing activity that would be anticipated in the presence of mutant PABPN1. |
| | BB-301 has been evaluated in prior non-clinical studies in animals that express mutant PABPN1 and, as a result, manifest the symptomatic phenotype of OPMD; in the symptomatic animal model of OPMD (i.e. the A17 mouse model), the achievement of PABPN1 silencing levels of 31% inhibition (or higher) following BB-301 administration led to resolution of OPMD disease symptoms and the elimination of the histopathological hallmarks of OPMD. |
Figure 8. PABPN1 Silencing (i.e., target knock-down) Achieved by BB-301 within Pharyngeal Muscle Tissues
There are key methodological distinctions between the current BB-301 Pilot Dosing Study conducted by Benitec as compared to the prior BB-301 Beagle dog dosing study carried out independently by the previous BB-301 licensee. The BB-301 dosing study conducted by the prior BB-301 licensee employed non-ideal routes and methods of BB-301 administration to the target pharyngeal muscle tissues and employed similarly limited analytical methods at the completion of the dosing phase of the study. Subsequently, the Benitec team worked to
17
Table of Contents
optimize the route and method of administration of BB-301 and to refine the core analytical methods employed following the completion of dosing of the large animal subjects.
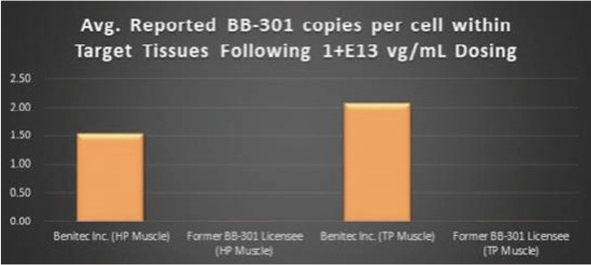
The current proprietary method of BB-301 delivery to the key pharyngeal muscles of study subjects, and the proprietary molecular analytical methods employed to assay the pharyngeal muscle tissues of study subjects, with both methods having been developed by the Benitec team, led to the observation of broad-based transduction of the targeted pharyngeal muscle tissues (Figure 9, represents individual sections of the TP muscle following BB-301 dosing). Critically, the Benitec-developed methods also facilitated the achievement of a 228-fold improvement (+22,647%) in BB-301 transduction of the HP muscle and a 113-fold improvement (+11,163%) in BB-301 transduction of the TP muscle relative to the levels of BB-301 transduction observed by the previous BB-301 licensee at identical BB-301 doses in identical canine study populations (Figure 10).
Figure 9. BB-301 Transduction Levels Achieved for Individual Sections of the TP Muscle Following BB-301 dosing
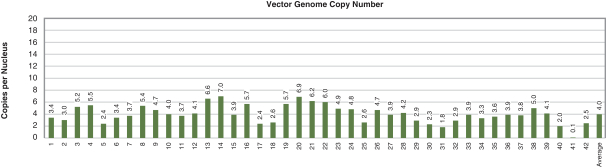
Figure 10. Impact of the Methodological Improvements to the BB-301 Large Animal Dosing Study Design on the Relative Pharyngeal Muscle Tissue Transduction Levels Achieved by Benitec vs. the Former BB-301 Licensee
18
Table of Contents
Following the disclosure of the positive interim BB-301 Pilot Dosing Study results, Benitec completed pre-CTA and pre-IND meetings with regulatory agencies in France, Canada, and the United States.
Summary of Regulatory Interactions:
| | Benitec successfully completed the regulatory interactions required to support initiation of the BB-301 clinical development program in 2022 |
| | Successful regulatory engagement comprised the completion of the following meetings: |
| | Pre-Clinical Trial Application (Pre-CTA) Consultation Meeting with Health Canada |
| | Scientific Advice Meeting with The National Agency for the Safety of Medicines and Health Products in France (LAgence nationale de sécurité du médicament et des produits de santé or ANSM) |
| | Type C Meeting with the U.S. Food and Drug Administration (the FDA) |
Benitec will begin the clinical development program for BB-301 in 2022.
Summary of the BB-301 Clinical Development Program:
| | The BB-301 clinical development program will begin in 2022, and the conduct of the development program will comprise approximately 76-weeks of follow-up for each OPMD study participant, inclusive of: |
| | 6-month pre-treatment observation periods employing quantitative radiographic imaging techniques for evaluation of the baseline disposition and natural history of OPMD-derived dysphagia in each study participant |
| | 1 day of BB-301 dosing to initiate participation in the Phase 1b/2a single-arm, open-label, sequential, dose escalation cohort study |
| | 52-weeks of post-dosing follow-up for conclusive evaluation of the primary and secondary endpoints of the Phase 1b/2a BB-301 treatment study |
| | The OPMD Natural History Study will begin in the second half of 2022, and this study will facilitate the characterization of OPMD patient disposition at baseline and assess subsequent rates of progression of dysphagia (swallowing impairment) in subjects with OPMD via the use of quantitative radiographic measures of global swallowing function and pharyngeal constrictor muscle function along with clinical assessments and patient-reported self-assessments of swallowing function |
| | Videofluoroscopic Swallowing Studies (VFSS) will be conducted to complete the following methodological assessments: |
| | Dynamic Imaging Grade of Swallowing Toxicity Scale (DIGEST) |
| | Pharyngeal Area at Maximum Constriction (PhAMPC) |
| | Pharyngeal Constriction Ratio (PCR) |
| | Clinical measures of global swallowing capacity and oropharyngeal dysphagia will be carried out |
| | Patient-reported measures of oropharyngeal dysphagia will be assessed |
| | The natural history of dysphagia observed for each OPMD study participant, as characterized by the quantitative radiographic measures and the clinical and patient self-reported assessments outlined above, will serve as the baseline for comparative assessments of safety and efficacy of BB-301 upon rollover of OPMD study subjects from the Natural History Study into the Phase 1b/2a BB-301 treatment study |
19
Table of Contents
| | Upon the achievement of 6-months of follow-up in the Natural History Study, OPMD Natural History Study participants can become eligible for enrollment into the Phase 1b/2a treatment study with the investigational genetic medicine, BB-301, which uses an AAV9-based gene therapy approach for the treatment of OPMD-derived dysphagia |
| | This first-in-human (FIH) clinical trial will be a Phase 1b/2a, open-label, dose escalation study to evaluate the safety and clinical activity of intramuscular doses of BB-301 administered to the pharyngeal muscles of subjects with OPMD |
| | Upon rollover from the Natural History Study into the Phase 1b/2a BB-301 treatment study, the follow-up of OPMD study participants will continue for 52-weeks, and the primary endpoints (safety and tolerability) and secondary endpoints (comprising the quantitative radiographic measures of global swallowing function and pharyngeal constrictor muscle function, and the clinical and patient-reported assessments noted above) will be evaluated during each 90-day period following Day 1 (Day 1 represents the day of BB-301 intramuscular injection). |
Summary of Risk Factors
An investment in our securities involves a high degree of risk. Any of the factors set forth herein under Risk Factors and the risk factors included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 and any subsequent Quarterly Report on Form 10-Q may limit our ability to successfully execute our business strategy. You should carefully consider all of the information set forth in this prospectus and in the documents incorporated by reference herein and, in particular, should evaluate the specific factors set forth herein under Risk Factors and the risk factors included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 and any subsequent Quarterly Report on Form 10-Q in deciding whether to invest in our securities. These risk factors include, among others:
| | We have incurred significant losses since inception and anticipate that we will continue to incur losses for the foreseeable future. If we are unable to achieve or sustain profitability, the market value of our common stock will likely decline; |
| | We have never generated any revenue from product sales and may never be profitable; |
| | Even if this offering is successful, we will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain capital when needed may negatively impact our ability to continue as a going concern; |
| | Our auditors report expresses substantial doubt about our ability to continue as a going concern; |
| | Our product candidates are based on ddRNAi and silence and replace technology. Currently, no product candidates utilizing ddRNAi technology or silence and replace technology have been approved for commercial sale and our approach to the development of ddRNAi technology and silence and replace technology may not result in safe, effective or marketable products; |
| | We are early in our product development efforts and our lead product candidate, BB-301, is in preclinical development. We may not be able to obtain regulatory approvals for the commercialization of BB-301 or other product candidates; |
| | Issues that may impact delivery of our therapeutics to the cell could adversely affect or limit our ability to develop and commercialize product candidates; |
| | We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours; |
20
Table of Contents
| | If we are unable to obtain or protect sufficient intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products; |
| | You will experience immediate dilution as a result of this offering and may experience additional dilution in the future; |
| | There is no public market for the pre-funded warrants or the common warrants being offered by us in this offering; |
| | Holders of the pre-funded warrants or the common warrants offered hereby will have no rights as common stockholders with respect to the common stock underlying the pre-funded warrants or the common warrants, as applicable, until such holders exercise their pre-funded warrants or common warrants, as applicable, and acquire our common stock, except as otherwise provided in the pre-funded warrants or the common warrants, as applicable; |
| | Provisions of the common warrants and the pre-funded warrants offered by this prospectus could discourage an acquisition of us by a third party; |
| | The common warrants are speculative in nature; |
| | We may not receive any additional funds upon the exercise of the pre-funded warrants and the common warrants; |
| | Significant holders or beneficial holders of our common stock may not be permitted to exercise warrants that they hold; |
| | This offering could cause our stock price to fall, which could result in us being delisted from The Nasdaq Capital Market; |
| | Future sales of our common stock, or the perception that such sales may occur, could depress the trading price of our common stock; |
| | We have broad discretion in the use of the net proceeds we receive from this offering and may not use them effectively; and |
| | We do not have enough authorized shares of common stock to issue upon the exercise of all of the common warrants and we require Stockholder Approval of the Capital Event and the subsequent filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation to effect such Capital Event in order to have a sufficient number of shares of common stock available for issuance upon exercise of the Series 2 Common Warrants. There is no assurance that such Stockholder Approval will be obtained which could materially and adversely impact the rights of the Series 2 Common Warrant holders. |
COVID-19 Pandemic
COVID-19 has been declared a pandemic by the World Health Organization and has spread to nearly every country, including Australia and the United States. The impact of this pandemic has been, and will likely continue to be, extensive in many aspects of society, which has resulted in, and will likely continue to result in, significant disruptions to businesses and capital markets around the world. The extent to which the coronavirus impacts us will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the coronavirus and its variants, and the actions to contain the coronavirus or treat its impact, including the effectiveness and adoption of vaccines for the virus, among others.
Certain elements of our research and development efforts are conducted globally, including the ongoing development of our silence and replace therapeutic for the treatment of Oculopharyngeal Muscular Dystrophy
21
Table of Contents
(OPMD), and will be dependent upon our ability to initiate preclinical and clinical studies despite the ongoing COVID-19 pandemic.
As we continue to actively advance our development programs, including the completion of our ongoing Toxicology and Biodistribution study for BB-301, we are in close contact with our principal investigators and preclinical trial sites, which are primarily located in France, and are assessing the impact of COVID-19 on our studies and the expected development timelines and costs on an ongoing basis. In light of developments relating to the COVID-19 global pandemic since the beginning of the outbreak, the focus of healthcare providers and hospitals on fighting the virus, and consistent with the FDAs industry guidance for conducting clinical trials, we have experienced delays to the original timeline regarding the initiation and anticipated completion of the ongoing BB-301 Clinical Trial Application (CTA)-enabling and Investigational New Drug Application (IND)-enabling development work. The initiation of the BB-301 Pilot Dosing Study in Beagle dogs, which represents a key component of the CTA-enabling and IND-enabling work, was delayed by several months, however, the study has been completed without incident. In addition, the BB-301 GLP Toxicology and Biodistribution Study in Beagle dogs, which represents another key component of the CTA-enabling and IND-enabling work, is nearing completion. The acquisition of chemical reagents, biological reagents and laboratory supplies which are essential for the conduct of basic laboratory research, the conduct of nonclinical studies and the completion of GMP manufacturing of BB-301, has also become challenging due to the disruption of global supply chains inherent to the production of these materials. We will continue to evaluate the impact of the COVID-19 pandemic on our business and we expect to reevaluate the timing of our anticipated preclinical and clinical milestones as we learn more and the impact of COVID-19 on our industry becomes clearer.
We have also implemented a halt of non-essential business travel and a rotation system whereby staff work from home and attend the laboratory on designated days which may result in a reduction of laboratory work. As we transition our employees back to our premises, there is a risk that COVID-19 infections occur at our offices or laboratory facilities and significantly affect our operations. Additionally, if any of our critical vendors are impacted, our business could be affected if we become unable to procure essential equipment in a timely manner or obtain supplies or services in adequate quantities and at acceptable prices.
Corporate Information
We were incorporated as a Delaware corporation on November 22, 2019 and completed the re-domiciliation (the Re-domiciliation) on April 15, 2020. Our predecessor, Benitec Limited, was incorporated under the laws of Australia in 1995. Our common stock is traded on The Nasdaq Capital Market under the symbol BNTC. Our principal executive offices are located at 3940 Trust Way, Hayward, California 94545. Our telephone number is (510) 780-0819, and our Internet website is www.benitec.com. The information on, or that can be accessed through, our website is not part of this prospectus and is not incorporated by reference herein.
Implications of Being a Smaller Reporting Company
We are a smaller reporting company and will remain a smaller reporting company while either (i) the market value of our stock held by non-affiliates was less than $250 million as of the last business day of our most recently completed second fiscal quarter or (ii) our annual revenue was less than $100 million during our most recently completed fiscal year and the market value of our stock held by non-affiliates was less than $700 million as of the last business day of our most recently completed second fiscal quarter. We may rely on exemptions from certain disclosure requirements that are available to smaller reporting companies, including many of the same exemptions from disclosure requirements as those that are available to emerging growth companies, such as reduced disclosure obligations regarding executive compensation in our registration statements, prospectus and our periodic reports and proxy statements. For so long as we remain a smaller reporting company, we are permitted and intend to rely on exemptions from certain disclosure and other requirements that are applicable to other public companies that are not smaller reporting companies.
22
Table of Contents
| Common stock offered by us |
25,380,710 shares (or 29,187,816 shares, if the underwriters option to purchase additional shares is exercised in full). |
| Pre-funded warrants offered by us |
We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, pre-funded warrants in lieu of shares of common stock that would otherwise result in any such purchasers beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock. The exercise price of each pre-funded warrant will equal $0.0001 per share. Each pre-funded warrant will be exercisable upon issuance and will not expire prior to exercise. We are offering up to 25,380,710 shares of common stock and up to 25,380,710 pre-funded warrants, in the aggregate not exceeding more than a total of 25,380,710 shares of common stock and pre-funded warrants. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the pre-funded warrants. |
| Common warrants offered by us |
We are issuing to purchasers of shares of our common stock and/or pre-funded warrants in this offering a common warrant to purchase up to one share of our common stock for each share and/or pre-funded warrant purchased in this offering for a combined purchase price of $ for shares and accompanying common warrants and $ for pre-funded warrants and accompanying common warrants. Because a common warrant to purchase share(s) of our common stock is being sold together in this offering with each share of common stock and, in the alternative, each pre-funded warrant to purchase one share of common stock, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. |
| A portion of the common warrants, the Series 1 Common Warrants, will be exercisable on the date of closing at an exercise price of $ per share and will expire on the five-year anniversary of such closing. As more fully described in the Form of Common Warrant, which is filed as an exhibit to the registration statement of which this prospectus forms a part, the remainder of the common warrants that are not so exercisable on the date of closing, the Series 2 Common Warrants, will become exercisable at an exercise price of $ per share on the date on which we (a) receive Stockholder Approval for a Capital Event and (b) effect such Capital Event by filing with the Secretary of State of the State of Delaware a certificate |
23
Table of Contents
| of amendment to our amended and restated certificate of incorporation, and will expire on the five-year anniversary of the date such certificate of amendment becomes effective. See Description of Securities Common Warrants for a more detailed discussion. |
| No fractional shares of common stock will be issued in connection with the exercise of a common warrant. In lieu of fractional shares, we will round down to the next whole share. See Description of Securities Common Warrants. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the Series 1 Common Warrants. |
| Option to purchase additional shares and/or common warrants |
We have granted the underwriter a 30-day option to purchase an aggregate of up to 3,807,106 additional shares of our common stock and/or up to 3,807,106 additional common warrants to purchase 3,807,106 shares of our common stock from us at the public offering price per share of common stock and common warrant, less the underwriting discounts and commissions. The underwriter may exercise its option to acquire additional shares for the sole purpose of covering over-allotments. See Underwriting. |
Common stock outstanding prior to this offering 8,171,690 shares.
| Common stock to be outstanding immediately following this offering |
33,552,400 shares (or 37,359,506 shares, if the underwriters option to purchase additional shares is exercised in full) assuming we sell only shares of common stock in this offering and assuming none of the common warrants issued in this offering are exercised. |
| Use of proceeds |
We estimate that the net proceeds from this offering will be approximately $18,179,836 ($20,969,835 if the underwriters option to purchase additional shares and/or common warrants is exercised in full), based on an assumed public offering price per share of common stock and common warrant of $0.7880, the last reported sale price of our common stock on the Nasdaq Capital Market on September 8, 2022, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming we sell only shares of common stock in this offering and no exercise of the common warrants. We intend to use the net proceeds from this financing for the clinical development of BB-301, including the natural history lead-in study and the Phase 1b/2a BB-301 treatment study, for the continued advancement of development activities for other existing and new product candidates, for general corporate purposes, and for strategic growth opportunities. See Use of Proceeds. |
| Dividend policy |
For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. |
24
Table of Contents
| Risk factors |
An investment in our securities involves a high degree of risk. You should read the Risk Factors section of this prospectus, as well as those risk factors included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 and any subsequent Quarterly Report on Form 10-Q, for a discussion of factors to consider carefully before deciding to invest in our securities. |
| Nasdaq symbol |
BNTC. |
| No listing of pre-funded warrants or common warrants |
We do not intend to apply for listing of the pre-funded warrants or the common warrants on any national securities exchange or trading system. Without a trading market, the liquidity of the pre-funded warrants and common warrants will be extremely limited. |
The number of shares of common stock to be outstanding after this offering is based on 8,171,690 shares of common stock outstanding at September 2, 2022 and excludes as of such date the following:
| | shares of common stock that may be issued upon exercise of pre-funded warrants and common warrants issued in this offering; |
| | 738,064 shares of common stock issuable upon exercise of stock options outstanding as of September 2, 2022 at a weighted-average exercise price of $6.95 per share; and |
| | 107,095 shares of common stock issuable upon the exercise of warrants exercisable for shares of common stock outstanding as of September 2, 2022 at a weighted-average exercise price of $10.50 per share. |
Unless otherwise indicated, all information in this prospectus assumes no purchaser elects to purchase pre-funded warrants, no exercise of the common warrants and no exercise by the underwriter of its option to purchase additional shares and/or common warrants.
25
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated financial data as of and for the years ended June 30, 2022 and 2021 are derived from our audited consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, which is incorporated by reference herein. You should read this data together with our consolidated financial statements and related notes included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, which is incorporated by reference herein. Our historical results are not necessarily indicative of our future results, and are not necessarily indicative of the results that may be expected for any interim periods, or any future year or period.
| Year ended June 30, | ||||||||
| (in thousands, except share and per share amounts) | 2022 | 2021 | ||||||
| Statements of operations data: |
||||||||
| Revenues: |
||||||||
| Revenues from customers |
$ | 73 | $ | 59 | ||||
| Government research and development grants |
| | ||||||
|
|
|
|
|
|||||
| Total revenues |
73 | 59 | ||||||
|
|
|
|
|
|||||
| Operating Expenses: |
||||||||
| Royalties and license fees |
9 | 123 | ||||||
| Research and development |
11,272 | 7,020 | ||||||
| General and administrative |
6,646 | 6,512 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
17,927 | 13,655 | ||||||
|
|
|
|
|
|||||
| Loss from Operations |
(17,854 | ) | (13,596 | ) | ||||
| Other Income (Loss): |
||||||||
| Foreign currency transaction loss |
(232 | ) | (333 | ) | ||||
| Interest income (expense), net |
(32 | ) | (6 | ) | ||||
| Other income (expense), net |
(79 | ) | 37 | |||||
| Unrealized gain (loss) on investment |
(11 | ) | 16 | |||||
|
|
|
|
|
|||||
| Total other loss, net |
(354 | ) | (286 | ) | ||||
|
|
|
|
|
|||||
| Net Loss |
$ | (18,208 | ) | $ | (13,882 | ) | ||
|
|
|
|
|
|||||
| Other Comprehensive Income (Loss): |
||||||||
| Unrealized foreign currency translation gain |
210 | 498 | ||||||
|
|
|
|
|
|||||
| Total Other Comprehensive Income |
210 | 498 | ||||||
|
|
|
|
|
|||||
| Total Comprehensive Loss |
$ | (17,998 | ) | $ | (13,384 | ) | ||
|
|
|
|
|
|||||
| Net Loss |
$ | (18,208 | ) | $ | (13,882 | ) | ||
|
|
|
|
|
|||||
| Basic and Diluted Weighted-Average Number of Shares Outstanding |
8,171,690 | 4,295,416 | ||||||
|
|
|
|
|
|||||
| Basic and Loss Per Share of Common Stock |
$ | (2.23 | ) | $ | (3.23 | ) | ||
|
|
|
|
|
|||||
26
Table of Contents
| June 30, | ||||||||
| (in thousands) | 2022 | 2021 | ||||||
| Balance sheet data: |
||||||||
| Cash and cash equivalents |
$ | 4,062 | $ | 19,769 | ||||
| Working capital |
2,288 | 19,239 | ||||||
| Total assets |
5,973 | 21,379 | ||||||
| Total long-term liabilities |
559 | | ||||||
| Total stockholders equity |
2,882 | 20,010 | ||||||
27
Table of Contents
Investing in our securities involves a high degree of risk. You should carefully consider the risks and uncertainties described below, as well as those risk factors included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 and any subsequent Quarterly Report on Form 10-Q, together with all of the other information contained in this prospectus and incorporated by reference herein, including our consolidated financial statements and the related notes, before deciding to invest in our securities. The risks and uncertainties described below and in the documents incorporated by reference herein are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that affect us. If any of the following risks actually occurs, our business, financial condition, results of operations and prospects could be materially and adversely affected, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks Related to Our Financial Condition and Capital Requirements
Even if this offering is successful, we will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain capital when needed may significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any approved product candidates.
Developing ddRNAi products is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in preclinical studies and in future clinical trials and as we undertake preclinical studies of new product candidates.
As of June 30, 2022 and June 30, 2021, our cash and cash equivalents were $4.1 million and $19.8 million, respectively. We estimate that the net proceeds from this offering will be approximately $18,179,836 ($20,969,835 assuming the full exercise of the underwriters option to purchase additional common stock and/or common warrants), assuming a public offering price of $0.7880 per share of common stock and common warrant, the last reported sale price of our common stock on the Nasdaq Capital Market on September 8, 2022, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, and assuming we sell only shares of common stock in this offering and no exercise of the common warrants . We estimate that these net proceeds, together with our existing cash and cash equivalents, will be sufficient to fund our operations until approximately the fourth quarter of 2023. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government grants or other third-party funding, strategic alliances and licensing arrangements or a combination of these approaches. In addition, because the length of time and activities associated with successful development of our product candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. In any event, we will require additional capital to obtain regulatory approval for our product candidates and to commercialize any product candidates that receive regulatory approval.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our common stock to decline. If we incur indebtedness we may be required to agree to restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could compromise our ability to conduct our business. We could also seek financing through arrangements with collaborative partners at an earlier stage than would otherwise be desirable and we may be required to relinquish rights to some or all of our technologies or product candidates or otherwise agree to terms unfavorable to us.
28
Table of Contents
If we are unable to obtain funding on a timely basis or on acceptable terms, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any approved product candidates.
Our auditors report expresses substantial doubt about our ability to continue as a going concern.
Our independent auditors report on our June 30, 2022 consolidated financial statements included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 includes a statement expressing substantial doubt about our ability to continue as a going concern. We have recurring net losses, which have resulted in an accumulated deficit of $148.3 million as of June 30, 2022 and $130.1 million as of June 30, 2021. We have incurred a net loss of $18.2 million for the fiscal year ended June 30, 2022 and $13.9 million for the fiscal year ended June 30, 2021. At June 30, 2022 and June 30, 2021, we had cash and cash equivalents of $4.1 million and $19.8 million, respectively. The Company does not have adequate liquidity to fund its operations for the next 12 months without raising additional funds and the success of raising such additional capital is not solely within the control of the Company. These factors raise substantial doubt about our ability to continue as a going concern.
We will continue to seek to raise additional working capital through public equity, private equity or debt financings. If we fail to raise additional working capital, or do so on commercially unfavorable terms, it would materially and adversely affect our business, prospects, financial condition and results of operations, and we may be unable to continue as a going concern. Future reports from our independent registered public accounting firm may also contain statements expressing substantial doubt about our ability to continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms, if at all.
Risks Relating to this Offering
You will experience immediate dilution as a result of this offering and may experience additional dilution in the future.
The public offering price for shares of our common stock and common warrants (or pre-funded warrants and common warrants in lieu thereof) offered hereby will be substantially higher than the net tangible book value per share of our common stock immediately after this offering. If you purchase common stock and common warrants (or pre-funded warrants and common warrants in lieu thereof) in this offering, you will incur substantial and immediate dilution in the net tangible book value of your investment. Net tangible book value per share represents the amount of total tangible assets less total liabilities, divided by the number of shares of our common stock then outstanding. To the extent that options that are currently outstanding are exercised, there will be further dilution to your investment. Exercise of the pre-funded warrants or any of the common warrants will also dilute your investment. We may also issue additional common stock, options and other securities in the future that may result in further dilution of your shares of our common stock.
There is no public market for the pre-funded warrants or the common warrants being offered by us in this offering.
There is no established public trading market for the pre-funded warrants or the common warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants or the common warrants on any national securities exchange or other nationally recognized trading system, including The Nasdaq Capital Market. Without an active market, the liquidity of the pre-funded warrants or the common warrants will be limited.
Holders of the pre-funded warrants or the common warrants offered hereby will have no rights as common stockholders with respect to the common stock underlying the pre-funded warrants or the common warrants, as applicable, until such holders exercise their pre-funded warrants or common warrants, as applicable, and acquire our common stock, except as otherwise provided in the pre-funded warrants or the common warrants, as applicable.
Until holders of the pre-funded warrants or the common warrants acquire our common stock upon exercise of the pre-funded warrants or the common warrants, as applicable, holders of pre-funded warrants or common
29
Table of Contents
warrants will have no rights with respect to the common stock underlying such pre-funded warrants or common warrants, except to the extent that holders of such pre-funded warrants or common warrants will have certain rights to participate in distributions or dividends paid on our common stock as set forth in the pre-funded warrants or the common warrants, as applicable. Further, as described elsewhere herein, certain of the common warrants that we are offering, the Series 2 Common Warrants, will not be exercisable until we obtain Stockholder Approval to effect a Capital Event, which we cannot guarantee, in which case such common warrant holders will not be able to exercise their common warrants at all. Upon exercise of the pre-funded warrants or the common warrants, as applicable, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date. See the Risk Factor titled, We do not have enough authorized shares of common stock to issue shares upon the exercise of all of the common warrants offered hereunder and we require Stockholder Approval of the Capital Event and the subsequent filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation to effect such Capital Event in order to have a sufficient number of shares of common stock available for issuance upon exercise of all of the common warrants. There is no assurance that such Stockholder Approval will be obtained which could materially and adversely impact the rights of the common warrant holders below for additional information.
Provisions of the common warrants and the pre-funded warrants offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the discussion of the provisions of our Certificate of Incorporation (as defined below), certain provisions of the common warrants and the pre-funded warrants offered by this prospectus could make it more difficult or expensive for a third party to acquire us. Such common warrants and pre-funded warrants prohibit us from engaging in certain transactions constituting fundamental transactions unless, among other things, the surviving entity assumes our obligations under the common warrants and the pre-funded warrants. Further, the common warrants and the pre-funded warrants provide that, in the event of certain transactions constituting fundamental transactions, with some exception, holders of the common warrants and the pre-funded warrants will have the right, at their option, to require us to repurchase such common warrants and pre-funded warrants at a price described in the common warrants and the pre-funded warrants. These and other provisions of the common warrants and the pre-funded warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
The common warrants are speculative in nature.
The common warrants offered hereby do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically, commencing on the date of an Initial Exercise Date, which date may vary as more fully described in the section titled Description of Securities Common Warrants, holders of the common warrants may acquire the common stock issuable upon exercise of such common warrants at an exercise price of $ per share. Moreover, following this offering, the market value of the common warrants is uncertain and there can be no assurance that the market value of the common warrants will equal or exceed their public offering price. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the common warrants and consequently, whether it will ever be profitable for holders of the common warrants to exercise the common warrants.
We may not receive any additional funds upon the exercise of the pre-funded warrants and the common warrants.
Each pre-funded warrant and common warrant may be exercised by way of a cashless exercise, meaning that the holder may not pay a cash purchase price upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the pre-funded warrant or the common warrant, as applicable. Accordingly, we may not receive any additional funds upon the exercise of the pre-funded warrants or the common warrants. In addition, the pre-funded warrants have an exercise price of $0.0001 per share of common stock, and as a result we will not receive significant additional funds upon their exercise even if not a cashless exercise.
30
Table of Contents
Significant holders or beneficial holders of our common stock may not be permitted to exercise warrants that they hold.
Holders of the pre-funded warrants and the common warrants will not be entitled to exercise any portion of any pre-funded warrant or common warrant which, upon giving effect to such exercise, would cause the aggregate number of shares of our common stock beneficially owned by the holder (together with its affiliates) to exceed a specified percentage of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants and the common warrants. As a result, you may not be able to exercise your pre-funded warrants or common warrants for shares of our common stock at a time when it would be financially beneficial for you to do so. In such circumstance, you could seek to sell your pre-funded warrants or common warrants to realize value, but you may be unable to do so in the absence of an established trading market for the pre-funded warrants or the common warrants.
We may fail to continue to meet the listing standards of The Nasdaq Capital Market whether or not this offering occurs. Even if this offering occurs, this offering could cause our stock price to fall, which could result in us being delisted from The Nasdaq Capital Market. Failure to maintain the listing of our common stock with a U.S. national securities exchange could adversely effect the liquidity off our common stock.
Our common stock is currently listed on The Nasdaq Capital Market. In order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including maintaining a minimum share price. For example, the current continued listing requirements of Nasdaq provide, among other things, that a company may be delisted if the bid price of its stock drops below $1.00 for a period of 30 consecutive business days.
On September 6, 2022, we received a letter from the Listing Qualifications Department of the Nasdaq Stock Market notifying us that the minimum bid price per share for our common stock fell below $1.00 for a period of 30 consecutive business days and that therefore we did not meet the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2). The letter also states that we will be provided 180 calendar days, or until March 6, 2023 to regain compliance with the minimum bid price requirement. In accordance with Rule 5810(c)(3)(A), we can regain compliance if at any time during the 180-day period the closing bid price of our common stock is at least $1.00 for a minimum of 10 consecutive business days. If by March 6, 2023, we cannot demonstrate compliance with the Rule 5550(a)(2), we may be eligible for additional time. To qualify for additional time, we will be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the bid price requirement, and we will need to provide written notice of our intention to cure the deficiency during the second compliance period. If we are not eligible for the second compliance period, then the Nasdaq Staff will provide notice that our securities will be subject to delisting. At such time, we may appeal the delisting determination to a Hearings Panel.
If we fail to satisfy the continued listing requirements of Nasdaq, such as the minimum share price requirement, Nasdaq may take steps to delist our securities. Such a delisting would likely have a negative effect on the price and liquidity of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we would take actions to restore our compliance with Nasdaqs listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our common stock from dropping below the Nasdaq minimum share price requirement or prevent future non-compliance with Nasdaqs listing requirements.
We intend to monitor the closing bid price of our common stock and may, if appropriate, consider implementing available options to regain compliance with the minimum bid price requirement. These options include completing a reverse stock split of our common stock for the purpose of meeting the closing bid price requirement.
31
Table of Contents
We intend to monitor the closing bid price of our common stock and may, if appropriate, consider implementing available options to regain compliance with the minimum bid price requirement. These options include completing a reverse stock split of our common stock for the purpose of meeting the closing bid price requirement. We have previously completed reverse stock splits in connection with complying with our listing requirement and any such option we undertake will not, in of itself, cause us to remain in compliance.
If our common stock were to be delisted from Nasdaq, our common stock could begin to trade on one of the markets operated by OTC Markets Group, including OTCQX, OTCQB or OTC Pink (formerly known as the pink sheets), as the case may be. In such event, our common stock could be subject to the penny stock rules which, among other things, require brokers or dealers to approve investors accounts, receive written agreements and determine investor suitability for transactions and disclose risks relating to investing in the penny stock market. Any such delisting of our common stock could have an adverse effect on the market price of, and the efficiency of the trading market for our common stock, not only in terms of the number of shares that can be bought and sold at a given price, but also through delays in the timing of transactions and less coverage of us by securities analysts, if any. Also, if in the future we were to determine that we need to seek additional equity capital, it could have an adverse effect on our ability to raise capital in the public or private equity markets. In addition, there can be no assurance that our common stock would be eligible for trading on any such alternative exchange or markets.
Future sales of our common stock, or the perception that such sales may occur, could depress the trading price of our common stock.
After the completion of this offering, we expect to have 33,552,400 shares (37,359,506 shares if the underwriter exercises its option to purchase additional shares in full) of our common stock outstanding, which may be resold in the public market immediately after this offering. We and all of our directors and executive officers have signed lock-up agreements for a period of 90 days following the date of this prospectus, subject to specified exceptions. See Underwriting.
The underwriter may, in its sole discretion and without notice, release all or any portion of the shares of our common stock subject to lock-up agreements. As restrictions on resale end, the market price of our common stock could drop significantly if the holders of these shares of our common stock sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our common stock or other securities.
We have broad discretion in the use of the net proceeds we receive from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds we receive in this offering, including for any of the purposes described in the section entitled Use of Proceeds, and you will not have the opportunity as part of your investment decision to assess whether our management is using the net proceeds appropriately. Because of the number and variability of factors that will determine our use of our net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business and cause the price of our common stock to decline. Pending their use, we may invest our net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders.
32
Table of Contents
We do not have enough authorized shares of common stock to issue shares upon the exercise of all of the common warrants offered hereunder and we require Stockholder Approval of the Capital Event and the subsequent filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation to effect such Capital Event in order to have a sufficient number of shares of common stock available for issuance upon exercise of all of the common warrants. There is no assurance that such Stockholder Approval will be obtained which could materially and adversely impact the rights of the common warrant holders.
We do not have enough shares of common stock currently authorized under our amended and restated certificate of incorporation to issue shares upon the exercise of all of the common warrants that we are issuing in this offering. Therefore, those common warrants that are not exercisable until Stockholder Approval is obtained, the Series 2 Common Warrants, will not be exercisable until we obtain such Stockholder Approval to effect an increase in our authorized shares of common stock or a reverse stock split in an amount sufficient to permit exercise in full of the common warrants and, thus, such shares of common stock underlying the Series 2 Common Warrants are not being registered in this offering. We currently have 40,000,000 shares of common stock authorized under our amended and restated certificate of incorporation. Of those authorized shares that remain available for issuance, we have reserved, in connection with this offering, an aggregate of 25,380,710 shares of common stock and an aggregate of 25,380,710 shares of common stock underlying the pre-funded warrants, all of which may be immediately exercisable; however we do not have enough authorized shares underlying all of the common warrants for each to be immediately exercised. Accordingly, only some of the common warrants, the Series 1 Common Warrants, may be immediately exercised and the remaining common warrants, the Series 2 Common Warrants, cannot be exercised unless and until the Capital Event is approved by stockholders and we effect such Capital Event by filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation and, thus, the shares of common stock underlying such Series 2 Common Warrants are not being registered in this offering. If our stockholders do not approve the Capital Event, we are obligated pursuant to the Form of Common Warrant (which is filed as an exhibit to the registration statement of which this prospectus forms a part) to continue to hold meetings of our stockholders to solicit approval, and 3,807,106 shares of common stock that may be issued if the underwriter exercises its over-allotment option until the Capital Event is approved. If we are unable to obtain such stockholder approval, the Series 2 Common Warrants will have no value and will expire. In no event may the warrants be net cash settled. There is no assurance we will be able to obtain such approval from our stockholders in which case the Series 2 Common Warrant holders would not be able to exercise their common warrants, which would materially and adversely impact the rights of such Series 2 Common Warrant holders.
We have used almost all of our unreserved, authorized shares.
After giving effect to this offering, we will have used almost all of our unreserved authorized shares and will need stockholder approval to implement an increase in our authorized shares of common stock or a reverse stock split. Our certificate of incorporation and the Delaware General Corporation Law, or the DGCL, currently require the approval of stockholders holding not less than a majority of all outstanding shares of capital stock entitled to vote in order to approve an increase in our authorized shares of common stock or a reverse stock split. There are no assurances that stockholder approval will be obtained, in which event will be unable to raise additional capital through the issuance of shares of common stock to fund our future operations.
33
Table of Contents
We estimate that the net proceeds from this offering will be approximately $18,179,836, or $20,969,835 if the underwriter exercises its option to purchase additional shares of common stock and/or common warrants in full, assuming a public offering price of $0.7880 per share of common stock and common warrant, the last reported sale price per share of our common stock on September 8, 2022 as reported on Nasdaq, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and assuming no sale of any pre-funded warrants in this offering and no exercise of the common warrants. The public offering price will be determined between us, the underwriter and investors based on market conditions at the time of pricing and may be at a discount to our current market price.
We will only receive additional proceeds from the exercise of the common warrants issuable in connection with this offering if such warrants are exercised at their exercise price of $ and the holders of such warrants pay the exercise price in cash upon such exercise. Such proceeds with respect to the common warrants could not exceed $ .
Each $0.10 increase or decrease in the assumed public offering price of $0.7880 per share of common stock and common warrant would increase or decrease the net proceeds from this offering by $2,360,406, assuming the number of shares and pre-funded warrants offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares and pre-funded warrants we are offering. An increase or decrease of 5,076,142 in the number of shares (or the common stock underlying the pre-funded warrants) offered by us, as set forth on the cover of this prospectus, would increase or decrease the net proceeds from this offering by approximately $3,720,000, assuming the assumed public offering price per share of common stock and common warrant remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We do not expect that a change by these amounts in either the public offering price per share of common stock and common warrant or in the number of shares offered by us would have a material effect on our use of the proceeds from this offering, although it may accelerate the time at which we will need to seek additional capital.
We intend to use the net proceeds from this financing for the clinical development of BB-301, including the natural history lead-in study and the Phase 1b/2a BB-301 treatment study, for the continued advancement of development activities for other existing and new product candidates, for general corporate purposes and for strategic growth opportunities.
The amount and timing of these expenditures will depend on a number of factors, including the progress of our research and development efforts, the progress of any partnering efforts, technological advances and the competitive environment for our product candidates. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the proceeds will be used in a way that does not yield a favorable, or any, return for us. Pending application of the net proceeds as described above, we intend to invest the proceeds in investment grade interest bearing instruments, or will hold the proceeds in interest bearing or non-interest bearing bank accounts.
34
Table of Contents
The following table sets forth our cash and cash equivalents and capitalization, each as of June 30, 2022:
| | on an actual basis; and |
| | on an as adjusted to give effect to the issuance of shares of common stock in the offering at an assumed public offering price of $0.7880 per share of common stock and common warrant, the last reported sale price per share of our common stock on September 8, 2022, as reported on Nasdaq, assuming no sale of any pre-funded warrants or exercise of any common warrants in this offering and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
You should consider this table in conjunction with Summary Consolidated Financial Data and our financial statements and the notes to those financial statements and the section titled Managements Discussion and Analysis of Financial Condition and Results of Operations included in our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, which are incorporated by reference herein. The as adjusted information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
| As of June 30, 2022 | ||||||||
| pro forma | ||||||||
| (in thousands) | Actual | As Adjusted(1) | ||||||
| Cash and cash equivalents |
$ | 4,062 | $ | 22,242 | ||||
| Total liabilities |
3,091 | 3,091 | ||||||
|
|
|
|
|
|||||
| Stockholders equity: |
||||||||
| Common stock, par value $0.0001 per share: |
||||||||
| 40,000,000 shares authorized; 8,171,690 actual; 33,552,400 pro forma |
1 | 4 | ||||||
| Additional paid-in capital |
152,453 | 170,630 | ||||||
| Accumulated deficit |
(148,327 | ) | (148,327 | ) | ||||
|
|
|
|
|
|||||
| Accumulated other comprehensive loss |
(1,245 | ) | (1,245 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders equity |
2,882 | 21,062 | ||||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 5,973 | $ | 24,153 | ||||
| (1) | The number of shares in the table above is based on 8,171,690 shares of common stock outstanding as of June 30, 2022 and does not include: |
| | shares of common stock that may be issued upon exercise of pre-funded warrants and common warrants issued in this offering; |
| | 738,064 shares of common stock issuable upon exercise of stock options outstanding as of June 30, 2022 at a weighted-average exercise price of $6.95 per share; and |
| | 107,095 shares of common stock issuable upon the exercise of warrants exercisable for shares of common stock outstanding as of June 30, 2022 at a weighted-average exercise price of $10.50 per share. |
Each $0.10 increase or decrease in the assumed public offering price of $0.7880 per share would increase or decrease, as applicable, our cash, additional paid-in capital, total stockholders equity and total capitalization by approximately $2,360,406, assuming that the number of shares offered by us remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each increase or decrease of 5,076,142 shares offered by us would increase or decrease the amount of our cash and total stockholders equity by approximately $3,720,000, assuming a public offering price of $0.7880 per share, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
35
Table of Contents
If you invest in our common stock and/or pre-funded warrants in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock after this offering. Our net tangible book value as of June 30, 2022 was approximately $2.1 million, or $0.26 per share of our common stock (based upon 8,171,690 shares of our common stock outstanding). Net tangible book value per share is equal to our total tangible assets less our total liabilities, divided by the number of shares of our outstanding common stock.
After giving effect to the sale of shares of our common stock and accompanying common warrants in this offering at the assumed public offering price of $0.7880 per share (the last reported sale price of our common stock on The Nasdaq Capital Market on September 8, 2022), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, and assuming we sell only shares of common stock in this offering and no exercise of the common warrants, our as adjusted net tangible book value as of June 30, 2022 would have been approximately $20.3 million, or $0.60 per share of common stock. This represents an immediate increase in as adjusted net tangible book value of $0.35 per share to our existing stockholders, and an immediate dilution of $0.18 per share to new investors purchasing securities in this offering at the assumed public offering price. The final public offering price will be determined between us, the underwriter and investors in the offering based on market conditions at the time of pricing and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final public offering price.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share and accompanying common warrant |
$ | 0.7880 | ||||||
| Historical net tangible book value per share as of June 30, 2022 |
$ | 0.26 | ||||||
| Pro forma increase in net tangible book value per share attributable to investors in this offering |
$ | 0.35 | ||||||
| As adjusted net tangible book value per share after giving effect to this offering |
$ | 0.60 | ||||||
| Dilution per share to investors participating in this offering |
$ | 0.18 |
A $0.10 increase in the assumed public offering price of $0.7880 per share, which is the last reported sale price of our common stock on The Nasdaq Capital Market on September 8, 2022, would result in an increase in our as adjusted net tangible book value per share after this offering by approximately $0.07 and the dilution per share to new investors purchasing shares in this offering by $0.03 assuming the number of securities offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. A $0.10 decrease in the assumed public offering price of $0.7880 per share, which is the last reported sale price of our common stock on The Nasdaq Capital Market on September 8, 2022, would result in a decrease in our as adjusted net tangible book value per share after this offering by approximately $0.07 and the dilution per share to new investors purchasing shares in this offering by $0.03 assuming the number of securities offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of securities to be issued in this offering. Each increase or decrease of 5,076,142 shares offered by us would increase or decrease our as adjusted net tangible book value per share and the dilution per share to new investors purchasing securities in this offering by $0.02 assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering as determined between us and the underwriter at pricing.
The foregoing discussion and table do not take into account further dilution to investors in this offering that could occur upon the exercise of outstanding options and warrants, including the pre-funded warrants and
36
Table of Contents
common warrants offered in this offering, having a per share exercise price less than the public offering price per share in this offering.
The discussion and table above are based on 8,171,690 shares of our common stock outstanding as of June 30, 2022, and do not include:
| | shares of common stock that may be issued upon exercise of pre-funded warrants and common warrants issued in this offering; |
| | 738,064 shares of common stock issuable upon exercise of stock options outstanding as of June 30, 2022 at a weighted-average exercise price of $6.95 per share; and |
| | 107,095 shares of common stock issuable upon the exercise of warrants exercisable for shares of common stock outstanding as of June 30, 2022 at a weighted-average exercise price of $10.50 per share. |
The discussion and table above assume no sale of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis.
To the extent that our outstanding options or warrants are exercised, new options are issued under our equity incentive plan, or additional shares of our common stock are issued in the future, there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
37
Table of Contents
MARKET PRICE OF OUR COMMON STOCK AND RELATED STOCKHOLDER MATTERS
Our common stock trades on Nasdaq under the symbol BNTC. We do not intend to apply for the listing of the pre-funded warrants or the common warrants that are part of this offering on any national securities exchange. On September 8, 2022, the closing sale price of our common stock as reported on Nasdaq was $0.7880 per share.
As of August 15, 2022, we had approximately 3,313 record holders of our common stock. The number of record holders is based on the actual number of holders registered on the books of our transfer agent and does not reflect holders of shares in street name or persons, partnerships, associations, corporations or other entities identified in security position listings maintained by depository trust companies.
We never have declared or paid any cash dividends on our capital stock. Currently, we anticipate that we will retain all available funds for use in the operation and expansion of our business and do not anticipate paying any cash dividends for the foreseeable future. Any future determination relating to our dividend policy will be made at the discretion of our Board and will depend on our future earnings, capital requirements, financial condition, prospects, applicable Delaware law, which provides that dividends are only payable out of surplus or current net profits, and other factors that our Board deems relevant.
38
Table of Contents
The following table sets forth certain information regarding the beneficial ownership of the Companys common stock as of August 15, 2022 by (i) each person or group of persons known by us to beneficially own more than five percent of our common stock, (ii) each of our named executive officers, (iii) each of our directors and (iv) all of our directors and executive officers as a group.
The following table gives effect to the shares of common stock issuable within 60 days of August 15, 2022 upon the exercise of all options and other rights beneficially owned by the indicated stockholders on that date. Beneficial ownership is determined in accordance with Rule 13d-3 promulgated under Section 13 of the Securities Exchange Act and includes voting and investment power with respect to shares. Percentage of beneficial ownership is based on 8,171,690 shares of common stock outstanding at the close of business on August 15, 2022. Except as otherwise noted below, each person or entity named in the following table has sole voting and investment power with respect to all shares of our common stock that he, she or it beneficially owns.
Unless otherwise indicated below, the address for each beneficial owner listed is c/o 3940 Trust Way, Hayward, California 94545.
| Name of Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
||||||||||
| Before Offering | After Offering | |||||||||||
| 5% or Greater Stockholders: |
||||||||||||
| Steven Michael Oliveira(1) |
1,000,000 | 12.2 | % | |||||||||
| Entities affiliated with Suvretta Capital Management, LLC(2) |
769,000 | 9.4 | % | |||||||||
| Entities affiliated with SilverArc Capital Management, LLC(3) |
587,499 | 7.2 | % | |||||||||
| Entities affiliated with Lincoln Park Capital, LLC(4) |
423,529 | 5.2 | % | |||||||||
| Directors and Named Executive Officers: |
||||||||||||
| Jerel A. Banks(5) |
128,114 | 1.6 | % | |||||||||
| Megan Boston(6) |
64,389 | * | ||||||||||
| J. Kevin Buchi(7) |
12,693 | * | ||||||||||
| Peter Francis(8) |
12,903 | * | ||||||||||
| Edward Smith(9) |
7,866 | * | ||||||||||
| All Executive Officers and Directors As a Group (5 persons)(10) |
225,965 | 2.8 | % | |||||||||
| * | Represents beneficial ownership of less than one percent of the Companys outstanding common stock. |
| (1) | Based on the information included in the Form 4 filed by Steven Michael Oliveira on December 9, 2021. The shares of common stock are held through Nemean Asset Management, LLC. The address of Steven Michael Oliveira is 225 Via Palacio, Palm Beach Gardens, FL, 33418. |
| (2) | Based on the information included in the Schedule 13G filed by Suvretta Capital Management, LLC (Suvretta) on May 7, 2021, Averill Master Fund, Ltd. (Averill) and Aaron Cowen. The address of the principal business office of Suvretta and Mr. Cowen is c/o Suvretta Capital Management, LLC, 540 Madison Avenue, 7th Floor, New York, New York 10022. The address of the principal business office of Averill is c/o Maples Corporate Services Limited, P.O. Box 309, Ugland House, Grand Cayman KY1-1104, Cayman Islands. |
| (3) | Based on information included in the Schedule 13G filed by SilverArc Capital Management, LLC (SilverArc) and Devesh Gandhi (Gandhi) on February 9, 2022. Gandhi is the Sole Member of SilverArc and as such is deemed to be the beneficial owner of these 587,499 shares. SilverArc Capital Alpha Fund I, L.P., a Delaware limited partnership for which SilverArc acts as an investment adviser, may be deemed to |
| beneficially own 33,017 of these 587,499 shares. SilverArc Capital Alpha Fund II, L.P., a Delaware limited partnership for which SilverArc acts as an investment adviser, may be deemed to beneficially own 384,277 of these 587,499 shares. Squarepoint Diversified Partners Fund Limited, a Cayman Island exempted |
39
Table of Contents
| company for which SilverArc acts as investment adviser, may be deemed to beneficially own 96,918 of these 587,499 shares. Atom Master Fund L.P., a Cayman Islands exempted limited partnership for which SilverArc acts as investment adviser, may be deemed to beneficially own 73,287 of these 587,499 shares. The address of the principal business office of SilverArc and Gandhi is 20 Park Plaza, 4th Floor, Boston, Massachusetts 02116. |
| (4) | Based on information included in the Schedule 13G filed by Lincoln Park Capital, LLC and its affiliates on April 28, 2021. Represents 423,529 shares held directly by Lincoln Park Capital Fund, LLC (LPC Fund). Alex Noah and Joshua B. Scheinfeld have shared voting and shared investment power over the shares held by LPC Fund. The address of the principal business office of Lincoln Park Capital, LLC and its affiliates is 440 North Wells, Suite 410, Chicago, Illinois 60654. |
| (5) | Represents stock options to acquire 128,114 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
| (6) | Includes 333 shares of common stock held by Boston Super Invest Pty A/C Boston Family Super that Megan Boston has sole voting power over and stock options to acquire 64,056 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
| (7) | Includes 4,827 shares of common stock held directly by Mr. Buchi and stock options to acquire 7,866 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
| (8) | Includes 4,737 shares of common stock held by the Francis Family Superannuation Fund, 300 shares of common stock held directly by Mr. Francis, and stock options to acquire 7,866 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
| (9) | Represents stock options to acquire 7,866 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
| (10) | Includes 10,197 shares of common stock and stock options to acquire 215,768 shares of common stock that have vested or will vest within 60 days of August 15, 2022. |
40
Table of Contents
The following description of our securities is intended as a summary only. We refer you to our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, amended and restated certificate of incorporation (the Certificate of Incorporation) and restated bylaws (the Bylaws), which are incorporated by reference into this prospectus, and to the applicable provisions of the Delaware General Corporation Law (DGCL). This description may not contain all of the information that is important to you and is subject to, and is qualified in its entirety by reference to, our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, our Certificate of Incorporation, our Bylaws and the applicable provisions of the DGCL. For information on how to obtain copies of our Annual Report on Form 10-K for the fiscal year ended June 30, 2022, our Certificate of Incorporation and our Bylaws, see Where You Can Find More Information.
General
Our authorized capital stock consists of 40,000,000 shares of our common stock, par value $0.0001 per share.
Common Stock
Dividend Rights. Subject to preferences that may be applicable to any then outstanding preferred stock, holders of the Companys common stock are entitled to receive dividends, if any, as may be declared from time to time by the Companys Board out of legally available funds. Dividends may be paid in cash, in property or in shares of common stock, subject to the provisions of the Certificate of Incorporation and applicable law. Declaration and payment of any dividend will be subject to the discretion of the Board. The time and amount of dividends will be dependent upon the Companys financial condition, operations, cash requirements and availability, debt repayment obligations, capital expenditure needs, restrictions in the Companys debt instruments, industry trends, the provisions of Delaware law affecting the payment of distributions to stockholders and any other factors the Board may consider relevant.
Voting Rights. Each holder of common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. The Companys stockholders do not have cumulative voting rights in the election of directors.
Liquidation Rights. In the event of the Companys liquidation, dissolution or winding up, holders of the Companys common stock are entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of the Companys debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences. Holders of the Companys common stock have no pre-emptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to the Companys common stock. The rights, preferences and privileges of the holders of the Companys common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that the Company may designate in the future.
Fully Paid and Nonassessable. All outstanding shares of the Companys common stock are fully paid and non-assessable.
Annual Stockholder Meetings. The Certificate of Incorporation and Bylaws provide that annual stockholder meetings will be held at a date, place (if any) and time, as exclusively selected by the Board. To the extent permitted under applicable law, the Company may but is not obligated to conduct meetings by remote communications, including by webcast.
41
Table of Contents
Common Warrants to be Issued as Part of this Offering
The following summary of certain terms and provisions of the common warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of common warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of common warrant for a complete description of the terms and conditions of the common warrants.
We are selling to investors in this offering shares of our common stock with accompanying common warrants to purchase 25,380,710 shares of our common stock and/or pre-funded warrants with accompanying common warrants to purchase 25,380,710 shares of our common stock at a combined purchase price of $ for shares and accompanying common warrants and $ for pre-funded warrants and accompanying common warrants.
Exercise Price and Duration. Each common warrant will be exercisable beginning on the Initial Exercise Date, which will either be the date of closing or, as applicable (and as more fully described below and in the Form of Common Warrant which is filed as an exhibit to the registration statement of which this prospectus forms a part), the date on which we (a) receive Stockholder Approval for a Capital Event and (b) effect such Capital Event by filing with the Secretary of State of the State of Delaware a certificate of amendment to our amended and restated certificate of incorporation, at an exercise price of $ per share, subject to adjustment. The common warrants will be exercisable for five years from their respective Initial Exercise Dates, but not thereafter. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Stockholder Approval for a Capital Event. Our authorized capital stock consists of 40,000,000 shares of our common stock, par value $0.0001 per share. We currently have 8,171,690 shares of our common stock issued and outstanding, an additional 1,911,744 shares of our common stock reserved for future issuance under our equity-based compensation plans, including 738,064 shares of our common stock that may be issued upon exercise of stock options outstanding pursuant to these plans and an additional 107,095 shares of our common stock reserved for future issuance upon exercise of warrants currently outstanding. As a result, after giving effect to this offering, including the exercise of the pre-funded warrants, we may only issue shares of our common stock unless and until we receive Stockholder Approval for a Capital Event. Consequently, of the common warrants, the Series 2 Common Warrants, will not be able to be exercised unless and until we receive such Stockholder Approval and effect such Capital Event by filing with the Secretary of State of the State of Delaware an amendment to our amended and restated certificate of incorporation and, thus, the shares of common stock underlying such Series 2 Common Warrants are not being registered in this offering. Pursuant to the Form of Common Warrant which is filed as an exhibit to the registration statement of which this prospectus forms a part, we are obligated to seek Stockholder Approval within of closing. If we are unable to obtain such stockholder approval, the Series 2 Common Warrants will have no value and will expire. In no event may the Series 2 Common Warrants be net cash settled.
Exercisability. Subject to limited exceptions, a holder of common warrants will not have the right to exercise any portion of its common warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to such exercise (the Beneficial Ownership Limitation); provided, however, that upon 61 days prior notice to the Company, the holder may increase or decrease the Beneficial Ownership Limitation, provided that in no event shall the Beneficial Ownership Limitation exceed 9.99%.
Cashless Exercise. The common warrants contain a cashless exercise feature that allows holders to exercise the common warrants without a cash payment to the Company upon the terms set forth in the common warrants, if, at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available for, the issuance of the shares to the exercising common warrant holder.
42
Table of Contents
Exercise Limitation. A holder (together with its affiliates) may not exercise any portion of a common warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common shares immediately after exercise, except that upon at least 61 days prior notice from the holder to us, the holder may increase the amount of beneficial ownership of outstanding stock after exercising the holders common warrants up to 9.99% of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the common warrants. Purchasers of common warrants in this offering may also elect prior to the issuance of the common warrants to have the initial exercise limitation set at 9.99% of our outstanding common shares.
Transferability. Subject to applicable laws, a common warrant may be transferred at the option of the holder upon surrender of the common warrant to us together with the appropriate instruments of transfer.
Fractional Shares. No fractional common shares will be issued upon the exercise of the common warrants. Rather, the number of common shares to be issued will be rounded down to the nearest whole number.
Trading Market. There is no established public trading market for the common warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the common warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the common warrants will be limited.
Right as a Stockholder. Except as otherwise provided in the common warrants or by virtue of such holders ownership of shares of our common stock, the holders of the common warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their common warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the common warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the common warrants will be entitled to receive upon exercise of the common warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the common warrants immediately prior to such fundamental transaction. In lieu of such consideration, a holder of common warrants may instead elect to receive a cash payment based upon the Black-Scholes value of their common warrants.
Amendment and Waiver. A common warrant may be modified or amended or the provisions thereof waived with the written consent of our company and the holder of the common warrant.
Pre-Funded Warrants to be Issued as Part of this Offering
The following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Exercise Price and Duration. Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.0001 and will be exercisable for share of our common stock. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
43
Table of Contents
Exercisability. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). Purchasers of the pre-funded warrants in this offering may elect to deliver their exercise notice following the pricing of the offering and prior to the issuance of the pre-funded warrants at closing to have their pre-funded warrants exercised immediately upon issuance and receive shares of common stock underlying the pre-funded warrants upon closing of this offering. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common stock immediately after exercise, except that upon at least 61 days prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holders pre-funded warrants. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will round down to the next whole share.
Cashless Exercise. If, at the time a holder exercises its pre-funded warrants, a registration statement registering the issuance of the shares of common stock underlying the pre-funded warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Exercise Limitation. A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding common shares immediately after exercise, except that upon at least 61 days prior notice from the holder to us, the holder may increase the amount of beneficial ownership of outstanding stock after exercising the holders pre-funded warrants up to 9.99% of the number of our common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding common shares.
Transferability. Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
Fractional Shares. No fractional common shares will be issued upon the exercise of the pre-funded warrants. Rather, the number of common shares to be issued will be rounded down to the nearest whole number.
Trading Market. There is no established public trading market for the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants will be limited.
Right as a Stockholder. Except as otherwise provided in the pre-funded warrants or by virtue of such holders ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Fundamental Transaction. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock,
44
Table of Contents
the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
Amendment and Waiver. A pre-funded warrant may be modified or amended or the provisions thereof waived with the written consent of our company and the holder of the pre-funded warrant.
Warrants
In September of 2019, Benitec Limited issued warrants for the purchase of its ADSs. In connection with the Re-domiciliation, these warrants became obligations of the Company. Currently, there are warrants outstanding for the exercise of 107,095 shares of the Companys common stock at an exercise price of $10.50 per share. The exercise price is subject to appropriate adjustment in the event of certain share dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our shares of common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders. The warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and, at any time a registration statement registering the issuance of the shares underlying the warrants under the Securities Act is effective and available for the issuance of such shares (or there is an effective registration statement for the resale of such shares), by payment in full in immediately available funds for the number of shares purchased upon such exercise. If no such registration statement is currently effective at the time of an exercise, the warrants will be exercisable on a cashless basis. No fractional shares will be issued in connection with the exercise of a warrant. In lieu of fractional shares, we will round up to the next whole share or pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Subject to limited exceptions, holders of warrants do not have the right to exercise any portion of their warrants if the holder (together with such holders affiliates, and any persons acting as a group together with such holder or any of such holders affiliates) would beneficially own a number of shares of common stock in excess of 4.99% of the shares of common stock then outstanding after giving effect to such exercise (the Warrant Beneficial Ownership Limitation); provided, however, that, upon notice to the Company, the holder may increase or decrease the Warrant Beneficial Ownership Limitation, provided that in no event shall the Warrant Beneficial Ownership Limitation exceed 9.99% and any increase in the Warrant Beneficial Ownership Limitation will not be effective until 61 days following notice of such increase from the holder to us.
Except as otherwise provided in the warrants or by virtue of such holders ownership of shares of common stock, the holder of a warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the warrant. The foregoing summary of the material terms and provisions of the warrants is qualified in its entirety by the form of warrant, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Anti-Takeover Effects of Provisions of the Certificate of Incorporation and Bylaws and DGCL
Some provisions of the DGCL, the Certificate of Incorporation and Bylaws could make the following transactions difficult: (i) acquisition of the Company by means of a tender offer; (ii) acquisition of the Company by means of a proxy contest or otherwise; or (iii) removal of incumbent officers and directors of the Company. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in the best interests of the Company, including transactions that might result in a premium over the market price for the Companys common stock.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of the Company to first negotiate with the Board.
45
Table of Contents
Delaware Anti-Takeover Statute. The Company is subject to Section 203 of the DGCL, which prohibits persons deemed interested stockholders from engaging in a business combination with a publicly-held Delaware corporation for three years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an interested stockholder is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporations voting stock, and a business combination includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the Board, such as discouraging takeover attempts that might result in a premium over the market price of the Companys common stock.
Special Stockholder Meetings. The Bylaws provide that a special meeting of stockholders may be called by (i) the Chairman of the Board, if any, (ii) the President or Chief Executive Officer, or (iii) the Board pursuant to a resolution adopted by a majority of the total number of directors then in office.
Requirements for Advance Notification of Stockholder Nominations and Proposals. The Bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors.
Composition of the Board of Directors; Election and Removal of Directors; Filling Vacancies
The Companys Board consists of five directors and the Board may, from time to time, fix the authorized number of directors by resolution of the Board. The Board is divided into three classes, designated Class I, Class II and Class III. Directors need not be stockholders of the Company.
Directors shall serve for a term ending on the date of the third annual meeting of stockholders following the annual meeting of stockholders at which such director was elected. The term of each director shall continue until the election and qualification of his or her successor and be subject to his or her earlier death, disqualification, resignation or removal. Except as otherwise provided by the DGCL, the Certificate of Incorporation or the Bylaws, directors shall be elected by a plurality of the votes of the shares present in person, by remote communication, if applicable, or represented by a duly authorized and executed proxy at the meeting and entitled to vote on the election of directors.
Subject to applicable law or by the Certificate of Incorporation, any director of the entire Board of the Company may be removed without cause by the affirmative vote of a majority of the holders of the Companys then-outstanding common stock entitled to vote generally at an election of directors. Furthermore, any vacancy on the Companys Board, however occurring, including a vacancy resulting from an increase in the size of the board, may be filled only by a majority vote of the Board then in office, even if less than a quorum, or by the sole remaining director.
Amendment of the Certificate of Incorporation and Bylaws. The Certificate of Incorporation may be amended in any manner permitted under the DGCL and the Bylaws may be amended by the vote or written consent of holders of a majority of the outstanding shares entitled to vote. The Board may also amend the Bylaws, other than a bylaw or amendment thereof specifying or changing a fixed number of directors or the maximum or minimum number or changing from a fixed to a variable board or vice versa.
Limitations of Liability and Indemnification Matters
Each of the Certificate of Incorporation and Bylaws provide that the Company is required to indemnify its directors and officers to the fullest extent not prohibited by Delaware law. The Bylaws also obligate the Company to advance expenses incurred by a director or officer in advance of the final disposition of any action
46
Table of Contents
or proceeding upon delivery to the Company of an undertaking, by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision, from which there is no further right to appeal, that such indemnitee is not entitled to be indemnified for such expenses.
To the fullest extent permitted by the DGCL, or any other applicable law, the Company, upon approval by the Board, may purchase insurance on behalf of any person required or permitted to be indemnified pursuant to the Bylaws.
Forum for Adjudication of Disputes
The Certificate of Incorporation provides that, unless the Company consents in writing to the selection of an alternative forum, the sole and exclusive forum for (i) derivative actions or proceedings brought on behalf of the Company, (ii) any action asserting a claim of breach of fiduciary duty owed by any director, officer or employee of the Company to the Company or the Companys stockholders, (iii) an action asserting a claim arising pursuant to any provision of the DGCL, or (iv) any action asserting a claim governed by the internal affairs doctrine shall be a state or federal court located within the state of Delaware. The Certificate of Incorporation further provides that the federal district courts of the United States of America shall, to the fullest extent permitted by law, be the exclusive forum for any complaint asserting a cause of action arising under the Securities Act.
Transfer Agent, Warrant Agent and Registrar
The transfer agent, warrant agent and registrar for the Companys common stock is Computershare Trust Company, N.A. The transfer agent, warrant agent and registrars address is 250 Royall St., Canton, Massachusetts 02021. Listing. Our common stock is listed on Nasdaq under the symbol BNTC.
47
Table of Contents
MATERIAL UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS FOR HOLDERS OF OUR COMMON STOCK, COMMON WARRANTS AND PRE-FUNDED WARRANTS
The following discussion is a summary of the material U.S. federal income tax considerations to U.S. Holders and Non-U.S. Holders (each as defined below and, together Holders) of the purchase, ownership and disposition of our common stock, common warrants and pre-funded warrants that are issued pursuant to this offering. This does not purport to be a complete analysis of all potential tax effects to Holders of purchasing, owning or disposing our common stock, common warrants or pre-funded warrants. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or foreign tax laws are not included in this discussion, and Holders should consult their own tax advisors as to these matters. This discussion is based on the Internal Revenue Code of 1986, as amended (the Code), final, temporary and proposed Treasury Regulations promulgated thereunder, judicial decisions and administrative pronouncements of the IRS, in effect as of the date of this offering. These authorities may change or be subject to differing interpretations. Any such change may be applied retroactively in a manner that could adversely affect a Holder. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance that the IRS will not take or that a court will not uphold a contrary position regarding the tax consequences of the purchase, ownership and disposition of our common stock, common warrants and pre-funded warrants.
This discussion is limited to Holders that hold our common stock, common warrants and pre-funded warrants as capital assets within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Holders particular circumstances. In addition, it does not address consequences relevant to Holders subject to special rules, including, without limitation:
| | banks, insurance companies and other financial institutions; |
| | real estate investment trusts, regulated investment companies, and other entities treated as conduits for U.S. federal income tax purposes; |
| | brokers, dealers or traders in securities; |
| | Holders who are subject to the mark-to-market accounting rules under Section 475 of the Code; |
| | controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; |
| | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes; |
| | tax-exempt organizations and governmental organizations or agencies; |
| | Holders who hold or receive our common stock, common warrants or pre-funded warrants pursuant to the exercise of any employee stock option or otherwise as compensation; |
| | tax-qualified retirement plans; |
| | qualified foreign pension funds as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds; |
| | U.S. expatriates and certain former citizens or long-term residents of the United States; |
| | U.S. Holders whose functional currency is not the United States dollar; |
| | Holders deemed to sell the our common stock, common warrants or pre-funded warrants under the constructive sale provisions of the Code; |
| | Holders for whom our common stock, common warrants and pre-funded warrants constitute qualified small business stock within the meaning of Section 1202 of the Code; |
48
Table of Contents
| | Holders subject to special tax accounting rules as a result of any item of gross income with respect to the stock being taken into account in an applicable financial statement (as defined in the Code); |
| | Holders holding our common stock, common warrants or pre-funded warrants as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; and |
| | Holders subject to the alternative minimum tax. |
If a partnership (or other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds our common stock, common warrants or pre-funded warrants, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock, common warrants or pre-funded warrants and the partners in such partnerships should consult their own tax advisors regarding the U.S. federal income tax consequences to them.
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT INTENDED AS TAX ADVICE. INVESTORS SHOULD CONSULT THEIR OWN TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK, COMMON WARRANTS OR PRE-FUNDED WARRANTS ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Characterization of the Pre-funded Warrants
Although the characterization of the pre-funded warrants for U.S. federal income tax purposes is not entirely clear, because the exercise price of the pre-funded warrants is a nominal amount, the Company expects to treat the pre-funded warrants as common stock of the Company for U.S. federal income tax purposes. Except where noted, the remainder of this discussion assumes that the pre-funded warrants will be so treated. Each Holder should consult its own tax advisor regarding the proper characterization of the pre-funded warrants for U.S. federal, state and local, and non-U.S. tax purposes, and the consequences to them of such treatment given their individual circumstances. Some portions of the below discussion make reference to potential consequences associated with the purchase, ownership and disposition of the pre-funded warrants independent of their potential characterization as common shares.
Allocation of Purchase Price
Each purchaser of shares of our common stock or pre-funded warrants must allocate its purchase price for such shares or pre-funded warrants between each share or pre-funded warrant, as applicable and the accompanying common warrant based on the respective relative fair market values of each at the time of issuance. This allocation of the purchase price will establish the Holders initial tax basis for U.S. federal income tax purposes for each share of our common stock, pre-funded warrant and common warrant. A Holders allocation of the purchase price among the shares of our common stock, pre-funded warrants and common warrants is not binding on the IRS or the courts, and no assurance can be given that the IRS or the courts will agree with a Holders allocation. Each Holder should consult its tax advisor regarding the allocation of the purchase price among the shares of our common stock, pre-funded warrants and common warrants.
U.S. Holders
For purposes of this discussion, a U.S. Holder is any beneficial owner of Securities that is, for U.S. federal income tax purpose For purposes of this discussion, a U.S. Holder is any beneficial owner of Securities that is, for U.S. federal income tax purposes:
| | an individual who is a citizen or resident of the United States; |
49
Table of Contents
| | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| | a trust that (i) is subject to the primary supervision of a U.S. court and the control of one or more U.S. persons (within the meaning of Section 7701(a)(30) of the Code), or (ii) has made a valid election under applicable Treasury Regulations to continue to be treated as a U.S. person. |
If you are not a U.S. Holder, this section does not apply to you. Please see the discussion under Non-U.S. Holders below.
Distributions on Common Stock
As described in the section captioned Dividend Policy, we do not anticipate declaring or paying distributions to Holders of our common stock in the foreseeable future. However, distributions (including constructive distributions as described below), if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles.
Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. Holders adjusted tax basis in our common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of the common stock and will be treated as described under U.S. HoldersSale or Other Taxable Disposition of Common Stock, Common Warrants or Pre-funded Warrants below.
Dividends we pay to a U.S. Holder that is a taxable corporation generally will qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided the common shares are held for more than 60 days during the 121-day period beginning 60 days before the ex- dividend date and certain other holding period requirements are met, dividends we pay to a non-corporate U.S. Holder generally will constitute qualified dividends that will be subject to tax at the maximum tax rate accorded to long-term capital gains. Dividends paid by us will generally be treated as income from U.S. sources. U.S. Holders should consult their own tax advisors regarding the holding period and other requirements that must be satisfied in order to qualify for the reduced maximum tax rate on dividends.
Sale or Other Taxable Disposition of Common Stock, Common Warrants or Pre-funded Warrants
Upon a sale, exchange or other taxable disposition of common stock, common warrants or pre-funded warrants, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holders adjusted tax basis in the common stock, common warrant or pre-funded warrant, as the case may be. A U.S. Holders adjusted tax basis in the common stock generally will equal the U.S. Holders acquisition cost of such security less, in the case of common stock (and potentially a pre-funded warrant and/or a common warrant) and as described further above, the amount of any prior distributions treated as a return of capital on such stock. If a U.S. Holder purchases or sells common stock, common warrants and/or pre-funded warrants together in a single transaction in which the purchase price for each of the common stock, common warrants and/or pre-funded warrants was not separately stated, the U.S. Holder generally would be required to allocate the purchase price among the subject securities so acquired or disposed of, as applicable, based on the relative fair market values of each (at the time of the acquisition or disposition, as applicable). U.S. Holders who purchase or sell common stock, common warrants and/or pre-funded warrants in a single transaction should consult with their tax advisors regarding such allocation.
Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. Holders holding period for the common stock, common warrants or pre-funded warrants disposed of exceeds one year. Long-term
50
Table of Contents
capital gains recognized by non-corporate U.S. Holders will be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Exercise of a Common Warrant or a Pre-funded Warrant
A U.S. Holder generally will not recognize taxable gain or loss on the acquisition of common stock upon exercise of a common warrant or pre-funded warrant. The U.S. Holders aggregate tax basis in the share of our common stock received upon exercise generally will be an amount equal to the sum of the U.S. Holders tax basis in the common warrant or pre-funded warrant prior to exercise and the common warrants or pre-funded warrants exercise price. Provided that a pre-funded warrant is treated as our common stock, a U.S. Holders holding period for the common stock received upon exercise of a pre-funded warrant will include the holding period for the pre-funded warrant. On the other hand, in the case of a common warrant, and also if the pre-funded warrant is treated as an option to purchase our stock, a U.S. Holders holding period for the common stock received upon exercise will begin on the date following the date of exercise of the common warrant (or the pre-funded warrant, as applicable) and will not include the period during which the U.S. Holder held the common warrant (or the pre-funded warrant, as applicable).
A U.S. Holder may be permitted to undertake a cashless exercise of common warrants or pre-funded warrants into our common stock. The U.S. federal income tax treatment of a cashless exercise is unclear, and the tax consequences of a cashless exercise could differ from the consequences of an exercise described above. For example, the cashless exercise could be treated as a taxable disposition of a portion of the common warrants, pre-funded warrants or the common shares into which they are exercisable. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise.
Lapse of Common Warrants
Upon the lapse or expiration of a common warrant, a U.S. Holder will recognize a loss in an amount equal to such U.S. holders tax basis in the common warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the common warrant is held for more than one year. The deductibility of capital losses is subject to limitations.
Certain Adjustments to the Common Warrants and Pre-funded Warrants, and Payments in Respect of the Common Warrants and Pre-funded Warrants
Under Section 305 of the Code, an adjustment to the number of shares of common stock that will be issued on the exercise of the common warrants or pre-funded warrants, or an adjustment to the exercise price of the common warrants or pre-funded warrants, may be treated as a constructive distribution to a U.S. Holder of the common warrants or pre-funded warrants if, and to the extent that, such adjustment has the effect of increasing such U.S. Holders proportionate interest in our earnings and profits or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of common warrants or pre-funded warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the U.S. Holder of the common warrants or pre-funded warrants generally should not be considered to result in a constructive distribution. Any constructive distribution would be treated as a dividend, return of capital or capital gain as described under the heading U.S. HolderDistributions on Common Stock above, and could be taxable whether or not there is an actual distribution of cash or other property. U.S. Holders should consult their tax advisors regarding the proper treatment of any adjustments to and payments in respect of common warrants or pre-funded warrants.
Net investment income tax
An additional 3.8% tax is imposed on the net investment income of non-corporate U.S. Holders, and on the undistributed net investment income of certain estates and trusts. Among other items, net investment income generally includes dividends paid on our common stock and certain net gain from the sale or other
51
Table of Contents
taxable disposition of our common stock, common warrants and pre-funded warrants, less certain deductions. U.S. Holders should consult their own tax advisors concerning the potential effect, if any, of this tax on holding our common stock, common warrants and pre-funded warrants in such U.S. Holders particular circumstances.
Backup withholding and information reporting
For non-corporate U.S. Holders, information reporting requirements, on IRS Form 1099, generally will apply to:
| | dividend payments or other taxable distributions on our common stock, common warrants and pre-funded warrants made to the non-corporate U.S. Holder within the United States or by a United States payor; and |
| | the payment of proceeds to the non-corporate U.S. Holder from the sale of a share of common stock, common warrants or pre-funded warrants effected at a United States office of a broker or through certain U.S.-related financial intermediaries. |
Additionally, backup withholding may apply to such payments if the non-corporate U.S. Holder:
| | fails to provide an accurate taxpayer identification number; |
| | is notified by the IRS that it has failed to report all interest and dividends required to be shown on its U.S. federal income tax returns; or |
| | in certain circumstances, fails to comply with applicable certification requirements. |
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-corporate U.S. Holders U.S. federal income tax liability (if any), provided the required information is timely furnished to the IRS. U.S. Holders are urged to consult their own tax advisors regarding the application of backup withholding and the availability of and procedure for obtaining an exemption from backup withholding in their particular circumstances.
Non-U.S. Holders
For purposes of this discussion, a Non-U.S. Holder is a beneficial owner of our common stock, common warrant or pre-funded warrants that, for U.S. federal income tax purposes, is neither a U.S. Holder (as defined above) nor a partnership or other pass-through entity. If you are not a Non-U.S. Holder, this section does not apply to you.
Distributions on Common Stock
As described in the section captioned Dividend Policy, we do not anticipate declaring or paying distributions to Holders of our common stock in the foreseeable future. However, distributions, if any, on our common stock in cash will generally constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a Non-U.S. Holders adjusted tax basis in its common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under Non-U.S. HoldersSale or Other Taxable Disposition of Common Stock, Common Warrants or Pre-Funded Warrants.
Subject to the discussion below regarding backup withholding and payments made to certain foreign accounts, dividends paid to a Non-U.S. Holder of our common stock (including constructive distributions treated as a dividend) that are not effectively connected with the Non-U.S. Holders conduct of a trade or business within the United States will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate as may be specified by an applicable income tax treaty).
52
Table of Contents
Non-U.S. Holders may be entitled to a reduction in or an exemption from withholding on dividends as a result of either (i) qualifying for the benefits of an applicable income tax treaty or (ii) the Non-U.S. Holder holding our common stock in connection with the conduct of a trade or business within the United States and dividends being paid in connection with that trade or business. To claim such a reduction in or exemption from withholding, the Non-U.S. Holder must provide the applicable withholding agent with a properly executed (i) IRS Form W-8BEN or W-8BEN-E (or applicable successor form) claiming an exemption from or reduction of the withholding tax under the benefit of an applicable income tax treaty, (ii) IRS Form W-8ECI (or applicable successor form) stating that the dividends are effectively connected with the conduct by the Non-U.S. Holder of a trade or business within the United States, or (iii) a suitable substitute form, as may be applicable. These certifications must be provided to the applicable withholding agent prior to the payment of dividends and must be updated periodically. Non-U.S. Holders that do not timely provide the applicable withholding agent with the required certification, but that qualify for a reduced rate under an applicable income tax treaty, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
Subject to the discussion below regarding backup withholding and payments made to certain foreign accounts, if dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holders conduct of a trade or business within the United States (or, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such dividends are attributable), then, although generally exempt from U.S. federal withholding tax (provided the Non-U.S. Holder provides appropriate certification, as described above), the Non-U.S. Holder will be subject to U.S. federal income tax on such dividends on a net income basis at the regular graduated U.S. federal income tax rates. In addition, a Non-U.S. Holder that is or is treated as a corporation for U.S. federal income tax purposes may be subject to an additional branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on its effectively connected earnings and profits for the taxable year that are attributable to such dividends, as adjusted for certain items. Non-U.S. Holders should consult their own tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Sale or Other Taxable Disposition of Common Stock, Common Warrants or Pre-funded Warrants
Subject to the discussion below regarding backup withholding and payments made to certain foreign accounts, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain recognized upon the sale or other taxable disposition of a share of our common stock, common warrants or pre-funded warrants unless:
| | the gain is effectively connected with the Non-U.S. Holders conduct of a trade or business within the United States (or, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such gain is attributable); |
| | the Non-U.S. Holder is a non-resident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| | we are or have been a U.S. real property holding corporation, or USRPHC, for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non-U.S. Holder held the common stock, common warrants or pre-funded warrants. |
Gain described in the first bullet point above will generally be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates. A Non-U.S. Holder that is a foreign corporation also may be subject to an additional branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
A Non-U.S. Holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate as may be specified by an applicable income tax treaty) on any gain derived from the sale or other taxable disposition, which may be offset by certain U.S. source capital losses of the Non-U.S.
53
Table of Contents
Holder (even though the individual is not considered a resident of the United States) provided the Non-U.S. Holder timely files U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe that we are not, and do not anticipate that we will become, a USRPHC.
The method of determining the amount of gain by a Non-U.S. Holder on disposition of the common stock, common warrants or pre-funded warrants generally will correspond to the method of determining the amount of gain (or loss) by a U.S. Holder on disposition of the common stock, common warrants or pre-funded warrants, as described under U.S. HoldersSale or Other Taxable Disposition of Common Stock, Common Warrants or Pre-funded Warrants above. Non-U.S. Holders should consult their own tax advisors regarding potentially applicable income tax treaties that may provide for different rules, and the potential application of other exceptions to these taxes.
Exercise of a Common Warrant or Pre-funded Warrant
For certain Non-U.S. Holders engaged in the conduct of a trade or business in the United States, the U.S. federal income tax treatment of the exercise of a common warrant or pre-funded warrant, generally will correspond to the U.S. federal income tax treatment of the exercise of a common warrant or pre-funded warrant by a U.S. Holder, as described under U.S. Holders Exercise of a Common Warrant or Pre-funded Warrant above. For all other Non-U.S. holders, the exercise of a common warrant or pre-funded warrant generally will not be a U.S. taxable event.
Lapse of a Common Warrant
Upon the lapse or expiration of a common warrant, a Non-U.S. Holder will recognize a loss in an amount equal to such Non-U.S. Holders tax basis in the common warrant. Any such loss generally will be a capital loss and will be long-term capital loss if the common warrant is held for more than one year. The deductibility of capital losses is subject to limitations.
Certain Adjustments to the Common Warrants and Pre-funded Warrants and Payments in Respect of the Common Warrants and Pre-funded Warrants
Under Section 305 of the Code, an adjustment to the number of shares of common stock that will be issued on the exercise of the common warrants or pre-funded warrants, or an adjustment to the exercise price of the common warrants or pre-funded warrants, may be treated as a constructive distribution to a Non-U.S. Holder of the common warrants or pre-funded warrants if, and to the extent that, such adjustment has the effect of increasing such Non-U.S. Holders proportionate interest in our earnings and profits or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of common warrants or pre-funded warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the Non-U.S. Holder of the common warrants or pre-funded warrants generally should not be considered to result in a constructive distribution. Any constructive distribution would be treated as a dividend, return of capital or capital gain as described under the heading Non-U.S. HoldersDistributions on Common Stock above, and could be taxable whether or not there is an actual distribution of cash or other property.
In addition, regulations governing dividend equivalents under Section 871(m) of the Code may apply to the common warrants or pre-funded warrants. Under those regulations, an implicit or explicit payment under the common warrants or pre-funded warrants that references a dividend distribution on our common stock (including an adjustment to the amount due on the common warrant or pre-funded warrant to take into account a dividend distribution on our common stock) would be taxable to a Non-U.S. Holder as described under the
54
Table of Contents
heading Non-U.S. HoldersDistributions on Common Stock above. Such dividend equivalent amount would be taxable and subject to withholding whether or not there is actual payment of cash or other property, and the Company may satisfy any withholding obligations it has in respect of the common warrants or pre-funded warrants by withholding from other amounts due to the Non-U.S. Holder. Non-U.S. Holders are encouraged to consult their own tax advisors regarding the application of Section 871(m) of the Code to the common warrants or pre-funded warrants.
Information Reporting and Backup Withholding
Subject to the discussion below regarding payments made to certain foreign accounts, a Non-U.S. Holder generally will not be subject to backup withholding with respect to payments of dividends on our common stock we make to the Non-U.S. Holder, provided the applicable withholding agent does not have actual knowledge or reason to know such Holder is a U.S. person and the Holder certifies its non-U.S. status by providing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or other applicable certification (or applicable successor form), or otherwise establishes an exception. However, information returns will be filed with the IRS in connection with any dividends or other distributions on our common stock paid to the Non-U.S. Holder (including constructive distributions), regardless of whether any tax was actually withheld. Copies of these information returns may also be made available under the provisions of a specific treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established.
Information reporting and backup withholding may apply to the proceeds of a sale of a share of our common stock, common warrants or pre-funded warrants within the United States, and information reporting may (although backup withholding will generally not) apply to the proceeds of the sale of a share of our common stock, common warrants or pre-funded warrants outside the United States conducted through certain U.S.-related financial intermediaries, in each case, unless the beneficial owner certifies under penalty of perjury that it is a Non-U.S. person on IRS Form W-8BEN or other applicable form or successor form (and the payor does not have actual knowledge or reason to know that the beneficial owner is a U.S. person) or otherwise establishes an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holders U.S. federal income tax liability (if any), provided the required information is timely furnished to the IRS. Non-U.S. Holders are urged to consult their own tax advisors regarding the application of backup withholding and the availability of and procedure for obtaining an exemption from backup withholding in their particular circumstances.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under the provisions of the law generally known as the Foreign Account Tax Compliance Act, or FATCA, on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends (including constructive dividends) paid on our common stock, common warrants or pre-funded warrants, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of our common stock, common warrants or pre-funded warrants paid to a foreign financial institution or a non-financial foreign entity (each as defined in the Code), unless (i) the foreign financial institution undertakes certain diligence and reporting obligations, (ii) the non-financial foreign entity either certifies it does not have any substantial U.S. owners (as defined in the Code) or furnishes identifying information regarding each substantial U.S. owner, or (iii) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (i) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain specified U.S. persons or U.S.-owned foreign entities (each as defined in the Code), annually report certain information about such accounts and withhold 30% on payments to non-compliant foreign financial institutions and certain
55
Table of Contents
other account holders. An intergovernmental agreement between the United States and an applicable foreign country, or future Treasury Regulations or other guidance, may modify these requirements.
Under the applicable Treasury Regulations and guidance from the IRS, withholding under FATCA generally applies to payments of dividends (including constructive dividends) on our common stock, common warrants or pre-funded warrants. The FATCA withholding tax will apply to all withholdable payments without regard to whether the beneficial owner of the payment would otherwise be entitled to an exemption from imposition of withholding tax pursuant to an applicable tax treaty with the United States or U.S. domestic law. We will not pay additional amounts to Holders of our common stock, common warrants or pre-funded warrants in respect of any amounts withheld. Proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds from the sale or other disposition of common stock, common warrants or pre-funded warrants entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Prospective investors should consult their own tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock, common warrants and pre-funded warrants.
56
Table of Contents
JMP Securities LLC is acting as the representative of each of the underwriters named below. Subject to the terms and conditions set forth in an underwriting agreement among us and the underwriters, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the number of shares of common stock set forth opposite its name below.
| Underwriter |
Number of Shares and Warrants |
|||
| Total |
||||
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the securities sold under the underwriting agreement if any are purchased other than those shares and/or common warrants covered by the over-allotment option described below. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased.
We have agreed to indemnify the several underwriters against certain liabilities including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the securities, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the securities, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officers certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representative has advised us that the underwriters propose initially to offer the securities to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. After this public offering, the representative may change the public offering price and concession and discount to dealers.
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share and Warrant |
Without Option |
With Option Exercised in Full |
||||||||||
| Public offering price |
||||||||||||
| Underwriting discount |
||||||||||||
| Proceeds, before expenses, to us |
||||||||||||
We have agreed to pay the representatives out-of-pocket accountable expenses, including its legal fees, up to a maximum amount of $125,000. The estimated expenses of this offering payable by us, exclusive of the underwriting discount, are approximately $ .
Over-allotment Option
We have granted an option to the underwriters to purchase up to additional shares of our common stock at a price per share of $ and/or up to an additional warrants to purchase up to shares of
57
Table of Contents
common stock at a price per warrant of $ , less, in each case, the underwriting discount. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover any over-allotments. If the underwriters exercise this option, each will be obligated, subject to conditions contained in the underwriting agreement, to purchase a number of additional shares of our common stock and/or warrants proportionate to that underwriters initial amount reflected in the above table.
Right of First Refusal
In connection with this offering, we granted to JMP Securities LLC, for the twelve (12) month period following the commencement of sales of securities, a right of first refusal to act as sole lead manager, underwriter and/or placement agent for any and all future public or private equity, equity-linked, convertible and debt offerings (excluding commercial bank debt), with at least 50% of the economics provided to placement agents or underwriters, as applicable, during such twelve (12) month period by us, or any successor to or any subsidiary of our company subject to such procedures as agreed upon in the underwriting agreement entered into in connection with this offering.
No Sales of Similar Securities
We and each of our executive officers and directors have agreed with the underwriters not to offer, sell or otherwise dispose of any common stock or any securities convertible into or exercisable or exchangeable for or that represent the right to receive common stock or any rights to acquire common stock for a period of 90 days after the Company receives Stockholder Approval, with certain limited exceptions, without first obtaining the written consent of JMP Securities LLC, the representative of the underwriters. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly:
| | offer, pledge, sell or contract to sell any common stock; |
| | sell any option or warrant to purchase any common stock; |
| | purchase any option or warrant to sell any common stock; |
| | grant any option or warrant for the sale of any common stock; |
| | lend or otherwise transfer or dispose of any common stock; |
| | exercise any right with respect to the registration of any common stock or other securities; or |
| | enter into any swap or other agreement or transaction that transfers, in whole or in part, directly or indirectly, the economic consequence of ownership of any common stock whether any such swap, agreement or transaction is to be settled by the delivery of shares of common stock or other securities, in cash or otherwise. |
This lock-up provision applies to common stock and to securities convertible into or exchangeable or exercisable for or that represent the right to receive common stock. It also applies to common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
Listing on Nasdaq
Our common stock is listed on Nasdaq under the symbol BNTC. We do not intend to apply for the listing of the common warrants or the pre-funded warrants on any national securities exchange or other nationally recognized trading system, including Nasdaq.
Discretionary Sales
The underwriters do not expect to sell more than 5% of the shares sold in this offering in the aggregate to accounts over which they exercise discretionary authority.
58
Table of Contents
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of our shares of common stock is completed, SEC rules may limit underwriters and selling group members from bidding for and purchasing our common stock. However, the representative may engage in transactions that stabilize the price of the common stock, such as bids or purchases to peg, fix or maintain that price.
In connection with this offering, the underwriters may purchase and sell our common stock in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of shares of our common stock than they are required to purchase in this offering. Covered short sales are sales made in an amount not greater than the underwriters over-allotment option described above. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing shares of our common stock in the open market. In determining the source of shares of our common stock to close out the covered short position, the underwriters will consider, among other things, the price of shares of our common stock available for purchase in the open market as compared to the price at which they may purchase shares of our common stock through the over-allotment option. Naked short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of our common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering. Stabilizing transactions consist of various bids for or purchases of shares of our common stock made by the underwriters in the open market prior to the completion of this offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representative has repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
Similar to other purchase transactions, the underwriters purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. The underwriters may conduct these transactions on Nasdaq, in the over-the-counter market or otherwise.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representative will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution of Shares
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses by electronic means, such as e-mail. In addition, the underwriters may facilitate Internet distribution for this offering to certain of their Internet subscription customers. The underwriters may allocate a limited number of securities for sale to their online brokerage customers. An electronic prospectus is available on the Internet website maintained by the underwriters. Other than the prospectus in electronic format, the information on the underwriters websites is not part of this prospectus.
Other Relationships
In the ordinary course of their business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and
59
Table of Contents
financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Sales Outside the United States
No action has been or will be taken in any jurisdiction (except in the United States) that would permit a public offering of the common stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or the common stock in any jurisdiction where action for that purpose is required. Accordingly, the common stock may not be offered or sold, directly or indirectly, and neither of this prospectus nor any other offering material or advertisements in connection with the common stock may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Each of the underwriters may arrange to sell common stock offered by this prospectus in certain jurisdictions outside the United States, either directly or through affiliates, where they are permitted to do so.
Notice to Prospective Investors in Canada
These securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are: (i) accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions (NI 45-106) or Subsection 73.3(1) of the Securities Act (Ontario), and (ii) permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchasers province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchasers province or territory for particulars of these rights or consult with a legal advisor.
Under Canadian Securities Law, National Instrument 33-105 Underwriting Conflicts (NI 33-105) provides disclosure requirements with respect to certain potential conflicts of interest that may exist between an issuer and underwriters, dealers or placement agents, as the case may be. Pursuant to section 3A.3 of NI 33-105, we and the representative are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
We and the representative hereby notify prospective Canadian purchasers that: (a) we may be required to provide personal information pertaining to the purchaser as required to be disclosed in Schedule I of Form 45-106F1 under NI 45-106 (including its name, address, telephone number and the aggregate purchase price of any securities purchased), or personal information, which form 45-106F1 may be required to be filed by us under NI 45-106, (b) such personal information may be delivered to the Ontario Securities Commission, or the OSC, in accordance with NI 45-106, (c) such personal information is collected indirectly by the OSC under the authority granted to it under the securities legislation of Ontario, (d) such personal information is collected for the purposes of the administration and enforcement of the securities legislation of Ontario, and (e) the public official in Ontario who can answer questions about the OSCs indirect collection of such personal information is the administrative support clerk at the OSC, Suite 1903, Box 55, 20 Queen Street West, Toronto, Ontario M5H 3S8, Telephone: (416) 593-3684. Prospective Canadian purchasers that purchase securities in this offering will be
60
Table of Contents
deemed to have authorized the indirect collection of the personal information by the OSC, and to have acknowledged and consented to its name, address, telephone number and other specified information, including the aggregate purchase price paid by the purchaser, being disclosed to other Canadian securities regulatory authorities, and to have acknowledged that such information may become available to the public in accordance with requirements of applicable Canadian laws.
Upon receipt of this prospectus, each Canadian purchaser hereby confirms that it has expressly requested that all documents evidencing or relating in any way to the sale of the securities described herein (including for greater certainty any purchase confirmation or any notice) be drawn up in the English language only. Par la réception de ce document, chaque acheteur Canadien confirme par les présentes quil a expressément exigé que tous les documents faisant foi ou se rapportant de quelque manière que ce soit à la vente des valeurs mobilières décrites aux présentes (incluant, pour plus de certitude, toute confirmation dachat ou tout avis) soient rédigés anglais seulement.
61
Table of Contents
The validity of the securities offered in this prospectus will be passed upon for us by Proskauer Rose LLP, Los Angeles, California. Ellenoff Grossman & Schole LLP, New York, New York, is acting as counsel for the underwriter in connection with this offering.
The consolidated financial statements of Benitec Biopharma Inc. as of and for the years ended June 30, 2022 and 2021 incorporated by reference in this registration statement, have been audited by Baker Tilly US, LLP, an independent registered public accounting firm, as set forth in their report thereon incorporated by reference in this registration statement, in reliance upon such report and upon the authority of said firm as experts in accounting and auditing.
62
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the securities offered hereby, reference is made to the registration statement and the exhibits and schedules filed therewith. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and as such we refer you to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement and the exhibits and schedules filed therewith may be inspected without charge at the public reference room maintained by the SEC, located at 100 F Street N.E., Washington, D.C. 20549, and copies of all or any part of the registration statement may be obtained from such offices upon the payment of the fees prescribed by the SEC. Please call the SEC at 1-800-SEC-0330 for further information about the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The Internet address is www.sec.gov.
We are subject to the information and periodic reporting requirements of the Exchange Act, and we file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information are available for inspection and copying at the public reference room and website of the SEC referred to above. We maintain a website at www.benitec.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act with the SEC free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Prior to the Re-domiciliation, Benitec Limited was a foreign private issuer. Information concerning Benitec Limited, including its annual reports on Form 20-F and current reports on Form 6-K, is also available free of charge on our website. The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this prospectus, and any references to such website or any other website are inactive textual references only. You may also request a copy of these filings, at no cost, by writing us at 3940 Trust Way, Hayward, California 94545 or info@benitec.com or telephoning us at (510) 780-0819.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC permits us to incorporate by reference the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from the date those documents are filed.
We incorporate by reference the following documents listed below (excluding any document or portion thereof to the extent such disclosure is furnished and not filed):
| | Our Annual Report on Form 10-K for the fiscal year ended June 30, 2022 filed with the SEC on September 2, 2022; and |
| | Our Current Report on Form 8-K, filed with the SEC on September 7, 2022. |
63
Table of Contents
We also incorporate by reference all documents that we file with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act that are made after the initial filing date of the registration statement of which this prospectus is a part until this offering has been completed. All filings from the date of the initial registration statement and prior to effectiveness of the registration statement shall be deemed to be incorporated by reference into the prospectus. We are not, however, incorporating, in each case, any documents or information that we are deemed to furnish and not file in accordance with SEC rules.
You may request and obtain a copy of any of the filings incorporated herein by reference, at no cost, by writing or telephoning us at the following address or phone number:
Benitec Biopharma Inc.
3940 Trust Way
Hayward, California 94545
(510) 780-0819
info@benitec.com
64
Table of Contents
25,380,710 Shares of Common Stock or
Pre-funded Warrants to Purchase 25,380,710 Shares of Common Stock and Common Warrants to Purchase 25,380,710 Shares of Common Stock
Shares of Common Stock underlying the Pre-funded Warrants and Series 1 Common Warrants

$20,000,000
PRELIMINARY PROSPECTUS
JMP Securities
A CITIZENS COMPANY
, 2022
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution |
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of common stock and pre-funded warrants being registered. All amounts are estimates except for the SEC registration fee and the Financial Industry Regulatory Authority, or FINRA, filing fee.
| Item |
Amount to be paid |
|||
| SEC registration fee |
$ | 4,264.20 | ||
| FINRA filing fee |
$ | 7,400.00 | ||
| Printing and engraving expenses |
$ | 5,000.00 | ||
| Legal fees and expenses |
$ | 200,000.00 | ||
| Accounting fees and expenses |
$ | 55,000.00 | ||
| Transfer agent and warrant agent fees and expenses |
$ | 10,000.00 | ||
| Miscellaneous fees and expenses |
$ | 13,500.00 | ||
|
|
|
|||
| Total |
$ | 295,164.20 | ||
|
|
|
|||
| Item 14. | Indemnification of Directors and Officers |
We are incorporated under the laws of the State of Delaware. Section 102 of the Delaware General Corporation Law permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his or her duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit.
Section 145 of the Delaware General Corporation Law provides that a corporation has the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses (including attorneys fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
As permitted by the Delaware General Corporation Law, our amended and restated certificate of incorporation includes a provision to eliminate the personal liability of our directors for monetary damages for breach of their fiduciary duties as directors, subject to certain exceptions. In addition, our amended and restated certificate of incorporation and amended and restated bylaws provide that we are required to indemnify our officers and directors under certain circumstances, including those circumstances in which indemnification would otherwise be discretionary, and we are required to advance expenses to our officers and directors as incurred in connection with proceedings against them for which they may be indemnified.
II-1
Table of Contents
We have entered into indemnification agreements with our directors and executive officers. These indemnification agreements are not intended to deny or otherwise limit third-party or derivative suits against us or our directors or officers, but to the extent a director or officer were entitled to indemnity or contribution under our bylaws, the financial burden of a third-party suit would be borne by us, and we would not benefit from derivative recoveries against the director or officer. Such recoveries would accrue to our benefit but would be offset by our obligations to the director or officer under the indemnification agreement.
The underwriting agreement provides that the underwriter is obligated, under certain circumstances, to indemnify our directors, officers and controlling persons against certain liabilities, including liabilities under the Securities Act. Reference is made to the form of underwriting agreement to be filed as Exhibit 1.1 hereto.
We maintain directors and officers liability insurance for the benefit of our directors and officers.
| Item 15. | Recent Sales of Unregistered Securities |
Over the past three years, the Company and Benitec Limited have issued and sold to third parties the securities listed below without registering the securities under the Securities Act of 1933, as amended (the Securities Act). None of these transactions involved any public offering. All the securities listed below were sold through private placement either (i) outside the United States or (ii) in the United States to a limited number of investors in transactions not involving any public offering. As discussed below, we believe that each issuance of these securities was exempt from, or not subject to, registration under the Securities Act.
On September 30, 2019, Benitec Limited entered into a securities purchase agreement (SPA) with certain sophisticated and professional investors in the United States to issue 2,800,000 American Depositary Shares (ADSs), with each ADS representing 20 fully paid ordinary shares (which were converted to 186,666 shares of common stock as part of the Re-domiciliation), at a purchase price of US$0.70 per ADS, in a registered direct offering. The Investors were also issued warrants to purchase up to 412,890 ADSs (representing 27,526 shares of common stock after the Re-domiciliation) in aggregate, at a purchase price per warrant equal to US$0.6999 per ADS to be issued on exercise of the warrant (Pre-Funded Warrants). The Pre-Funded Warrants were exercisable at any time from issue, in whole or in part, at an exercise price of US$0.0001 per ADS issued on exercise (subject to certain adjustments), provided that the beneficial ownership of the relevant Investor in the total number of ADSs on issue not exceed 9.99%. The Pre-Funded Warrants have all been exercised. This issuance was exempt from registration under the Securities Act in reliance on Section 4(a)(2).
On April 15, 2020, the Company completed the Re-domiciliation. In connection with the Re-domiciliation, Benitec issued 1,070,957 shares of common stock, on the basis of one share of common stock for every 300 ordinary shares of Benitec Limited issued and outstanding prior to the Re-domiciliation. The Re-domiciliation was effected pursuant to a statutory scheme of arrangement under Australian law (the Scheme). The issuance of Benitecs shares of common stock in the Scheme was exempt from registration under the Securities Act in reliance on Section 3(a)(10).
On April 22, 2020, Benitec issued 37,417 shares of common stock in connection with a cashless exercise of warrants exercisable for 107,095 shares of common stock. The issuance was exempt from registration under the Securities Act in reliance on Section 3(a)(9).
| Item 16. | Exhibits and financial statement schedules |
(a) Exhibits
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
II-2
Table of Contents
| Item 17. | Undertakings |
The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the Calculation of Registration Fee table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(A) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(B) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
II-3
Table of Contents
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to any charter provision, by law or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act of 1933, each filing of the registrants annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plans annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(ii) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(I) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(iii) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Table of Contents
II-5
Table of Contents
| * | Filed herewith. |
| ** | Previously filed. |
| | Indicates a management contract or compensatory plan. |
II-6
Table of Contents
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Hayward, State of California, on September 8, 2022.
| BENITEC BIOPHARMA INC. | ||
| By: | /s/ Dr. Jerel Banks |
|
| Dr. Jerel Banks Chief Executive Officer |
||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date |
||
| /s/ Dr. Jerel Banks Dr. Jerel Banks |
Chief Executive Officer, Director (principal executive officer) |
September 8, 2022 | ||
| /s/ * Megan Boston |
Executive Director, Director (principal accounting and financial officer) |
September 8, 2022 | ||
| /s/ * J. Kevin Buchi |
Director | September 8, 2022 | ||
| /s/ * Peter Francis |
Director | September 8, 2022 | ||
| /s/ * Edward Smith |
Director | September 8, 2022 | ||
| *By: |
/s/ Dr. Jerel Banks |
|
| Dr. Jerel Banks Attorney-in-fact |
||
II-7