EX-99.2
Published on April 18, 2024

Exhibit 99.2 Research and Development Day Presentation April 2024 1 ©2024 Benitec Biopharma | All Rights Reserved

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of section 27A of the US Securities Act of 1933 and section 21E of the US Securities Exchange Act of 1934. Benitec has tried to identify such forward-looking statements by use of such words as expects, intends, hopes, anticipates, believes, could, may, evidences and estimates,” or the negative of these terms, and other similar expressions, but these words are not the exclusive means of identifying such statements. Such statements include, but are not limited to, any statements relating to Benitec's pipeline of ddRNAi-based therapeutics, the initiation, progress and outcomes of pre-clinical and clinical trials, the timing of the availability of data from our clinical trials, the timing and sufficiency of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and Benitec's product candidates, the intellectual property position and any other statements that are not historical facts. Such forward-looking statements involve risks and uncertainties, including, but not limited to, risks and uncertainties relating to the difficulties or delays in our plans to develop and potentially commercialize our product candidates, the timing of the initiation and completion of pre-clinical and clinical trials, the timing of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, potential future out-licenses and collaborations, our intellectual property position and duration of our patent portfolio, the ability to procure additional sources of financing, unanticipated delays, further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development, the ability to enroll sufficient numbers of subjects in clinical trials, determinations made by the US Food and Drug Administration and other governmental authorities, Benitec’s ability to protect and enforce its patents and other intellectual property rights, Benitec’s dependence on its relationships with its collaboration partners and other third parties, the efficacy or safety of Benitec’s products and the products of Benitec’s collaboration partners, the acceptance of Benitec’s products and the products of Benitec’s collaboration partners in the marketplace, market competition, sales, marketing, manufacturing and distribution requirements, greater than expected expenses, expenses relating to litigation or strategic activities, Benitec’s ability to satisfy its capital needs through increasing its revenue and obtaining additional financing, the impact of the COVID-19 pandemic, the disease caused by the SARS-CoV-2 virus and similar events, which may adversely impact Benitec’s business and pre-clinical and future clinical trials, the impact of local, regional, and national and international economic conditions and events, and other risks detailed from time to time in filings that Benitec makes with the US Securities and Exchange Commission, including our most recent annual report on Form 10-K and our reports on Form 8-K. Such statements are based on management's current expectations, but actual results may differ materially due to various factors, including those risks and uncertainties mentioned or referred to in this presentation. Accordingly, you should not rely on those forward-looking statements as a prediction of actual future results. Benitec disclaims any intent or obligation to update these forward-looking statements, except as required by law. 2 ©2024 Benitec Biopharma | All Rights Reserved

Research and Development 01 Introduction to S peakers Day Agenda Corporate Overview 02 03 OP MD Clinical and P athophys iological Overview 04 BB -301: Mechanis m of Action and Clinical Developm ent P rogram VF S S and S ubject-R eported Outcom e Meas ures 05 06 P relim inary B B -301 P has e 1b Clinical Data S um m ary Upcom ing Miles tones 07 3 ©2024 B enitec B iopharm a | All R ights R es erved

Professional Biography: Bernard Brais, MDCM, PhD, Professor, Department of Neurology and Neurosurgery at Montreal Neurological Institute (MNI), McGill University Health Centre (MGH, MNH) is Director of the Rare Neurological Diseases Group. He completed his MDCM, neurology residency and PhD at McGill. He is also trained as a historian of neurosciences and genetics. His research largely focuses on the genetic basis of neurogenetic disorders with founder effects in Quebec, with an increasing focus on disorders with ataxic manifestations such as Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS). Since 2007, he has headed a team of researchers on ARSACS. Dr. Brais has played important roles in identifying causal genes for Oculopharyngeal muscular dystrophy (OPMD), Hereditary Sensory and Autonomic Neuropathy type II (HSANII), Limb Girdle Muscular Dystrophy with Quadriceps atrophy (LGMD2L), Pol III-related leukodystrophies, and ZAK congenital myopathy. Bernard Brais, MDCM, PhD Professor, Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University Health Centre Disclosures: Clinical advisor and consultant to Benitec Biopharma Inc 4 ©2024 B enitec B iopharm a | All R ights R es erved

Professional Biography: Emily Plowman, PhD, CCC-SLP, FASHA, Professor, Department of Otolaryngology – Head and Neck Surgery, The Ohio State University College of Medicine is Director of the Aerodigestive Research Core across its two sites at the Ohio State University and University of Florida and Director of the Wexner Medical Center Dysphagia Program. She is an internationally recognized expert in the field of dysphagia who has held uninterrupted funding from the National Institutes of Health (NIH) since commencing her academic career in 2009. Her current research at OSU and UF are supported by the National Institute of Aging, National Institute of Nursing Research, National Institute of Cancer, Department of Defense, and the ALS Association. Dr. Plowman has authored over 85 peer-reviewed scientific manuscripts, given over 600 lectures worldwide, and obtained over 30 external research grants. In addition to her own research, Dr. Plowman is passionate about mentoring the future generation of clinician scientists and her mentorship efforts were recently recognized by the National Institutes of Health with the NINDS Story Landis Award for Outstanding Mentorship by a Neuroscientist (2022) and the University of Florida Doctoral Mentor of the Year award (2021). She was Emily Plowman, PhD, CCC-SLP, FASHA inducted into the American Speech and Hearing Association as a Fellow in 2022 and was Professor, Department of Otolaryngology - Head and Neck elected to be the incoming President of the international Dysphagia Research Society for Surgery, The Ohio State University College of Medicine 2026. Disclosures: Clinical advisor and consultant to Benitec Biopharma Inc 5 ©2024 B enitec B iopharm a | All R ights R es erved

Benitec Corporate Overview Jerel A. Banks, MD, PhD 6 ©2024 B enitec B iopharm a | All R ights R es erved

Corporate Highlights Novel Technology Platform • Benitec’s DNA-directed RNA interference (ddRNAi) platform combines gene therapy with RNA interference (RNAi) to simultaneously silence & replace disease-causing genes permanently, following a single administration • Platform has application in diseases that cannot be treated with gene silencing or gene therapy alone Lead Asset Entered Clinical Evaluation in Orphan Disease in November 2023 • BB-301 is being developed to treat dysphagia (difficulty swallowing) in subjects with Oculopharyngeal Muscular Dystrophy (OPMD). There are no therapies approved for the treatment of OPMD. The estimated prevalence in the US, Europe, Canada & Israel is 15k subjects. • Compelling preclinical data demonstrated complete restoration of muscle function in vivo via a murine disease model • The Investigational New Drug (IND) application for BB-301 was approved to proceed by the FDA in June 2023, and the first study subject was safely dosed in the BB-301 Phase 1b/2a clinical trial (NCT06185673) in November 2023 Significant Near-Term Milestones • Preliminary clinical safety data and clinical efficacy data for the BB-301 Phase 1b/2a clinical trial are expected in 2024 Seasoned Management Team • Benitec’s management team has broad expertise in gene therapy development, biological manufacturing and capital allocation 7 ©2024 Benitec Biopharma | All Rights Reserved

E xperienced and E fficient Management Jerel A. Banks, MD, PhD CEO & Executive Chairman Healthcare investment professional with over 15 years of experience Former Vice president & co-portfolio manager at Franklin Templeton Investments M.D., Ph.D. Brown University; A.B. Princeton University Megan Boston Executive Director CEO & managing director of multiple ASX-listed entities Chartered Accountant with over 20 years of experience Held senior executive roles at various banking institutions in risk and compliance as well as PricewaterhouseCoopers Claudia Kloth, PhD SVP of Manufacturing Over 20 years of cGMP manufacturing & process development experience in therapeutics Led process development group at Lonza Viral Therapeutics Developed, optimized, and transferred robust viral-based products (Ad5, AAV, lentivirus) to cGMP manufacturing Guided process transfer & validation activities of Yervoy (BMY) 8 ©2024 B enitec B iopharm a | All R ights R es erved

Oculopharyngeal Muscular Dystrophy: Clinical and Pathophysiological Overview Bernard Brais, MDCM, PhD 9 ©2024 Benitec Biopharma | All Rights Reserved
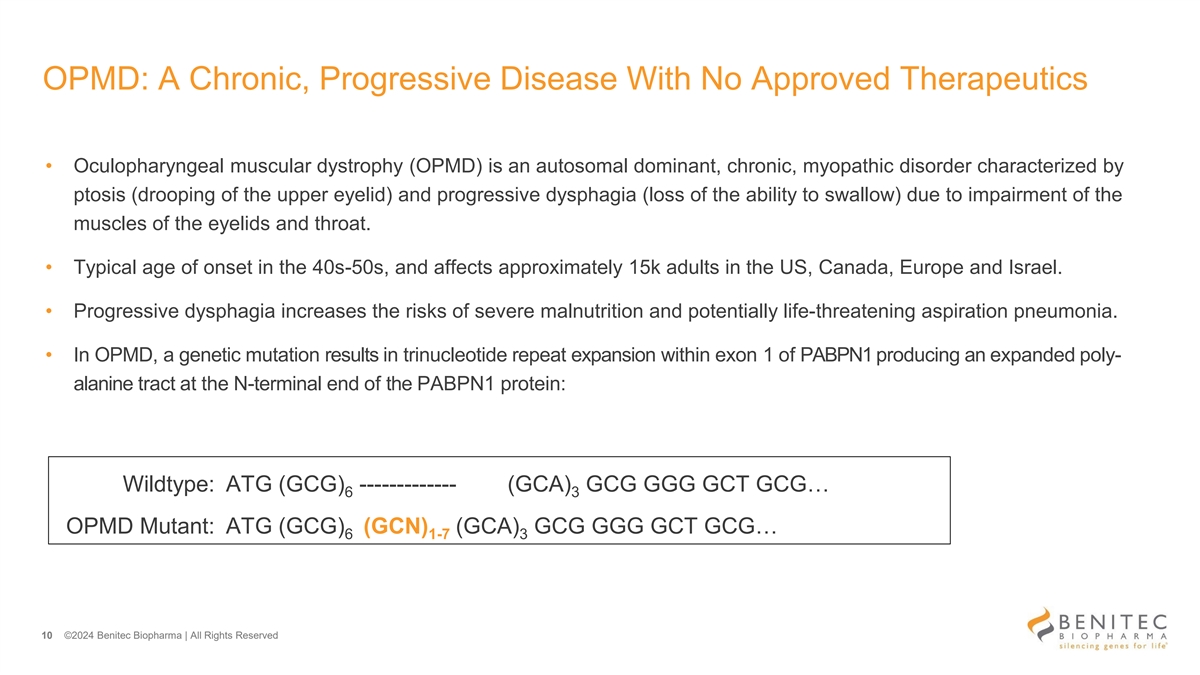
OPMD: A Chronic, Progressive Disease With No Approved Therapeutics • Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant, chronic, myopathic disorder characterized by ptosis (drooping of the upper eyelid) and progressive dysphagia (loss of the ability to swallow) due to impairment of the muscles of the eyelids and throat. • Typical age of onset in the 40s-50s, and affects approximately 15k adults in the US, Canada, Europe and Israel. • Progressive dysphagia increases the risks of severe malnutrition and potentially life-threatening aspiration pneumonia. • In OPMD, a genetic mutation results in trinucleotide repeat expansion within exon 1 of PABPN1 producing an expanded poly- alanine tract at the N-terminal end of the PABPN1 protein: Wildtype: ATG (GCG) ------------- (GCA) GCG GGG GCT GCG… 6 3 OPMD Mutant: ATG (GCG) (GCN) (GCA) GCG GGG GCT GCG… 6 1-7 3 10 ©2024 Benitec Biopharma | All Rights Reserved
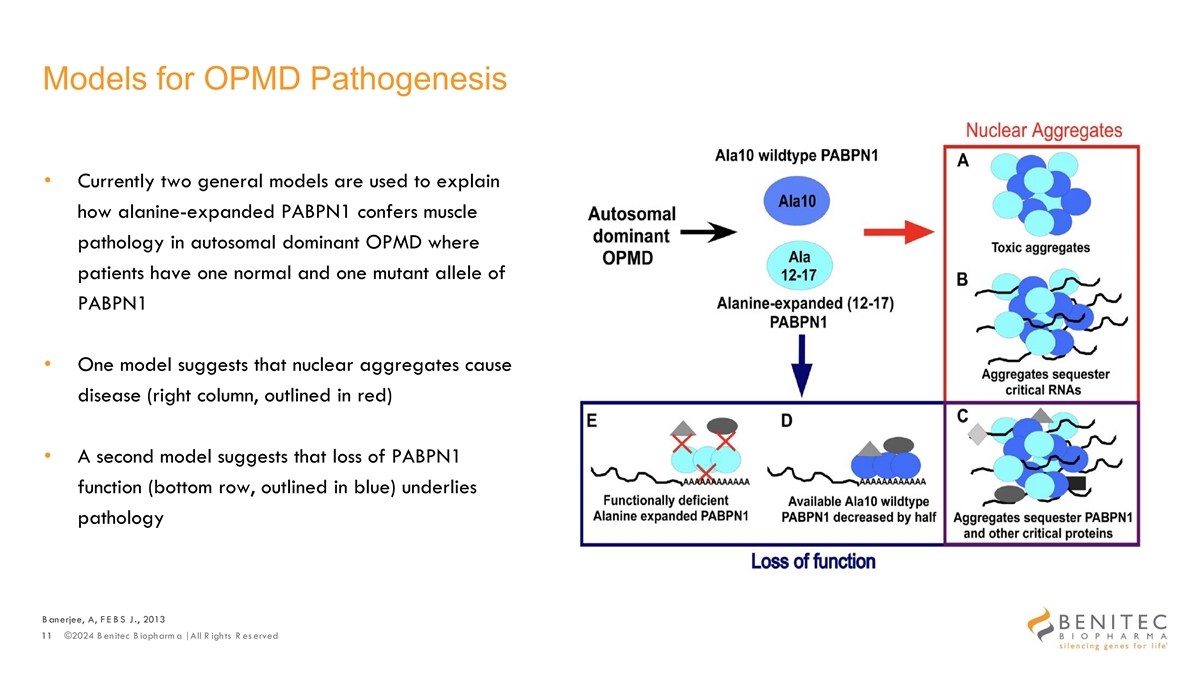
Models for OPMD Pathogenesis • Currently two general models are used to explain how alanine-expanded PABPN1 confers muscle pathology in autosomal dominant OPMD where patients have one normal and one mutant allele of PABPN1 • One model suggests that nuclear aggregates cause disease (right column, outlined in red) • A second model suggests that loss of PABPN1 function (bottom row, outlined in blue) underlies pathology B anerjee, A, F E B S J., 2013 11 ©2024 B enitec B iopharm a | All R ights R es erved
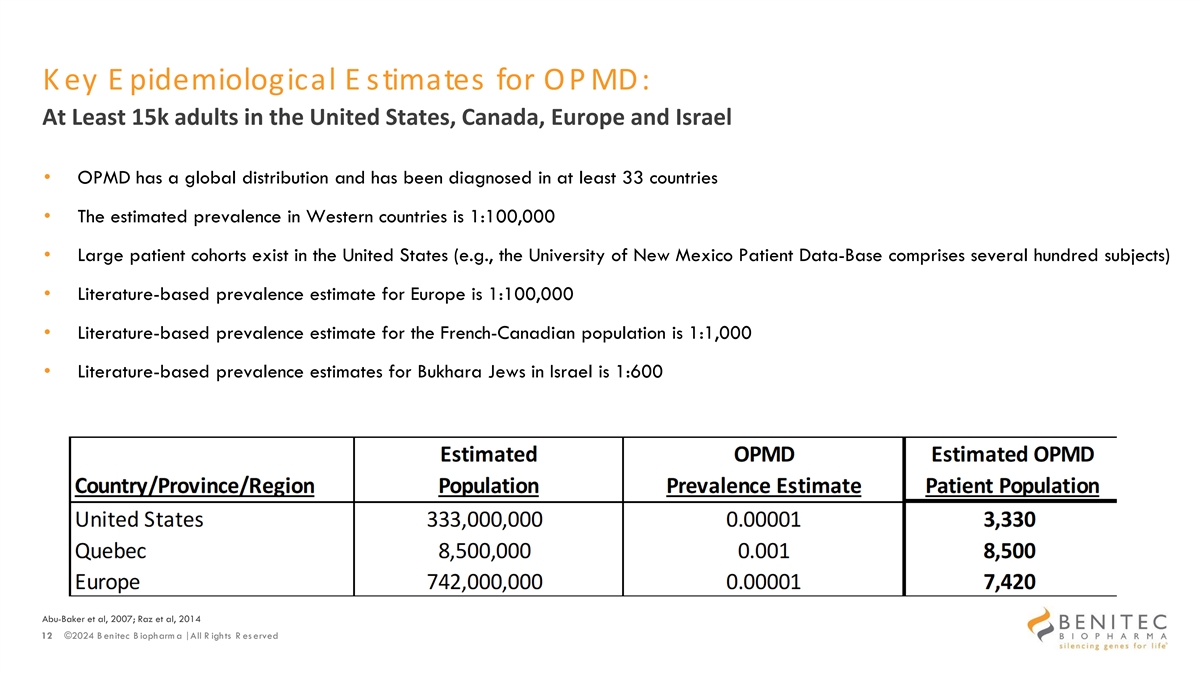
K ey E pidemiological E stimates for OP MD: At Least 15k adults in the United States, Canada, Europe and Israel • OPMD has a global distribution and has been diagnosed in at least 33 countries • The estimated prevalence in Western countries is 1:100,000 • Large patient cohorts exist in the United States (e.g., the University of New Mexico Patient Data-Base comprises several hundred subjects) • Literature-based prevalence estimate for Europe is 1:100,000 • Literature-based prevalence estimate for the French-Canadian population is 1:1,000 • Literature-based prevalence estimates for Bukhara Jews in Israel is 1:600 Abu-Baker et al, 2007; Raz et al, 2014 12 ©2024 B enitec B iopharm a | All R ights R es erved
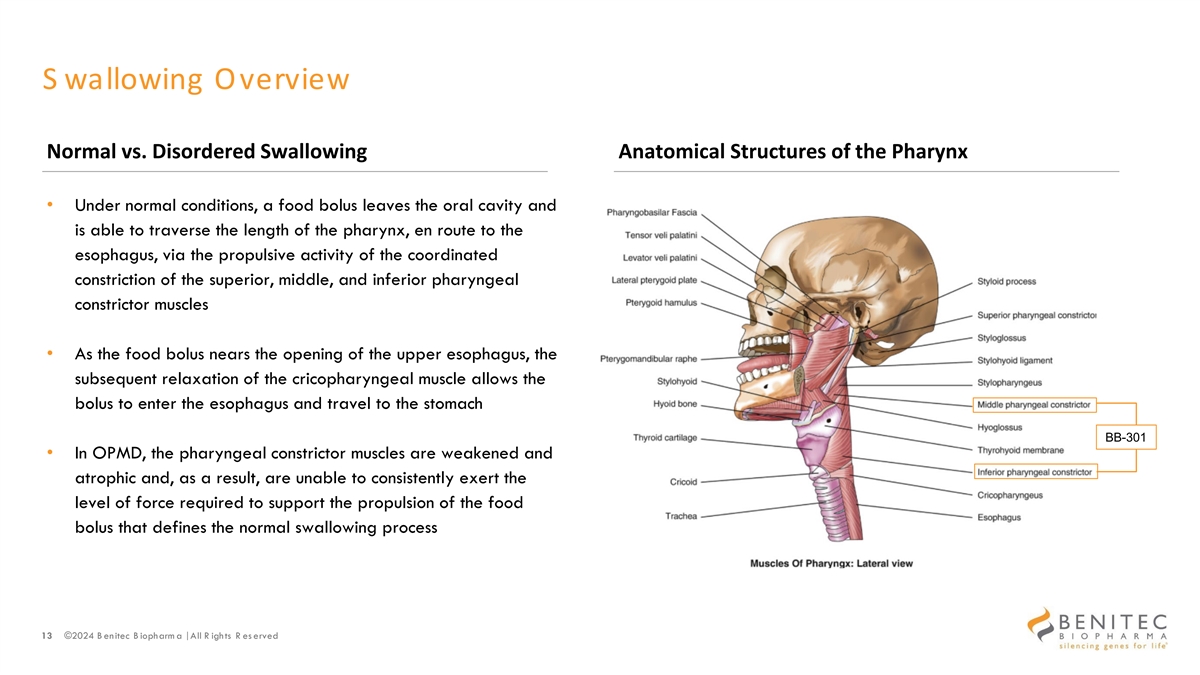
S wallowing Overview Normal vs. Disordered Swallowing Anatomical Structures of the Pharynx • Under normal conditions, a food bolus leaves the oral cavity and is able to traverse the length of the pharynx, en route to the esophagus, via the propulsive activity of the coordinated constriction of the superior, middle, and inferior pharyngeal constrictor muscles • As the food bolus nears the opening of the upper esophagus, the subsequent relaxation of the cricopharyngeal muscle allows the bolus to enter the esophagus and travel to the stomach BB-301 • In OPMD, the pharyngeal constrictor muscles are weakened and atrophic and, as a result, are unable to consistently exert the level of force required to support the propulsion of the food bolus that defines the normal swallowing process 13 ©2024 B enitec B iopharm a | All R ights R es erved

OPMD: Onset and Progression • More frequent choking • Prolonged mealtime • Avoidance of specific foods (e.g., rice, chicken, toast, etc.) • Drooping eyelids that are often asymmetrical at disease onset • Absence of limb weakness at disease onset 14 ©2024 B enitec B iopharm a | All R ights R es erved

OPMD: Clinical Presentation • A retrospective chart review was conducted at the Saguenay Neuromuscular Clinic (Quebec, Canada) • All health record of patients with an OPMD diagnosis were screened to identify patients who met inclusion criteria • Patients were excluded if another neurological or musculoskeletal disorder was present that could impact the evolution of the disease • Dysphagia was present in 96.6% of subjects • Pharyngeal pooling of thickened secretions was present in 74.1% of subjects • Dysphagia worsens over time and, as a result, patients can develop malnutrition and aspiration pneumonia which can lead to death Brisson, JD, Muscle & Nerve, 2020 15 ©2024 Benitec Biopharma | All Rights Reserved
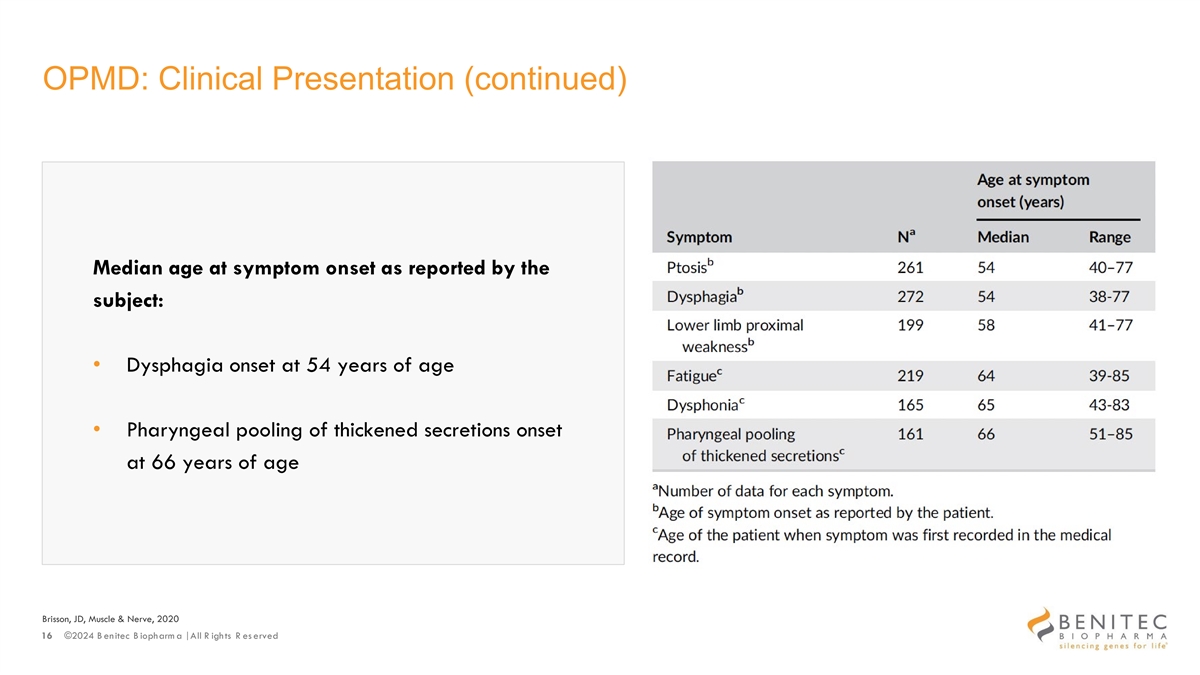
OPMD: Clinical Presentation (continued) Median age at symptom onset as reported by the subject: • Dysphagia onset at 54 years of age • Pharyngeal pooling of thickened secretions onset at 66 years of age Brisson, JD, Muscle & Nerve, 2020 16 ©2024 B enitec B iopharm a | All R ights R es erved
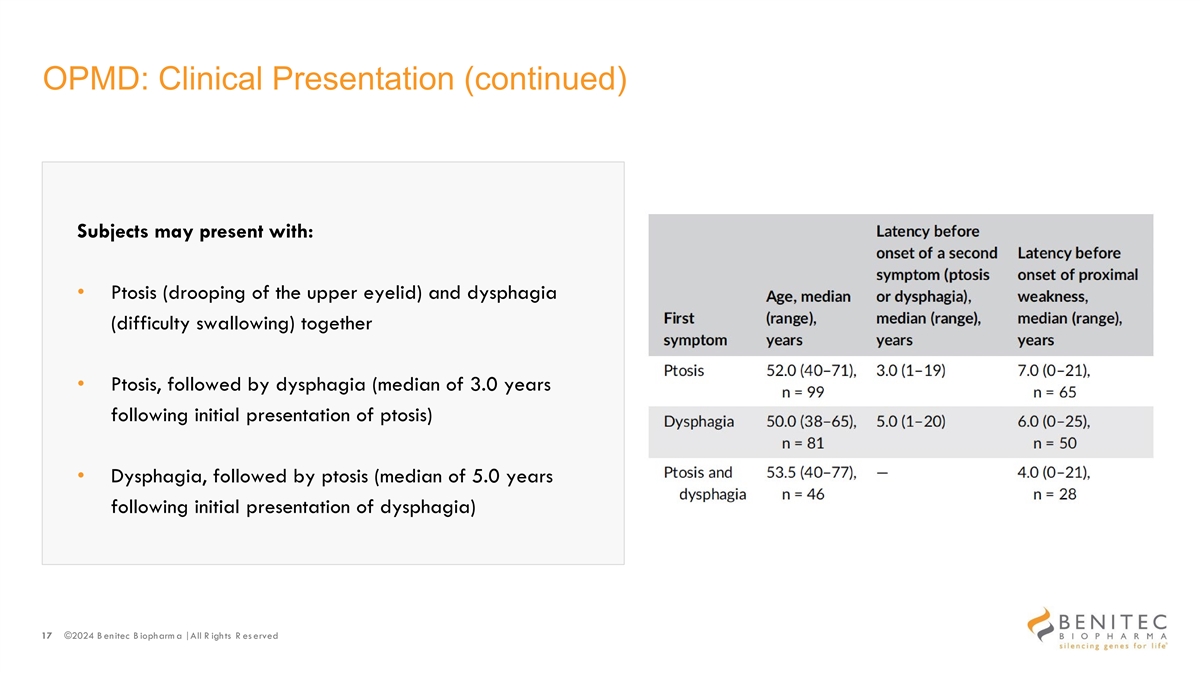
OPMD: Clinical Presentation (continued) Subjects may present with: • Ptosis (drooping of the upper eyelid) and dysphagia (difficulty swallowing) together • Ptosis, followed by dysphagia (median of 3.0 years following initial presentation of ptosis) • Dysphagia, followed by ptosis (median of 5.0 years following initial presentation of dysphagia) 17 ©2024 B enitec B iopharm a | All R ights R es erved
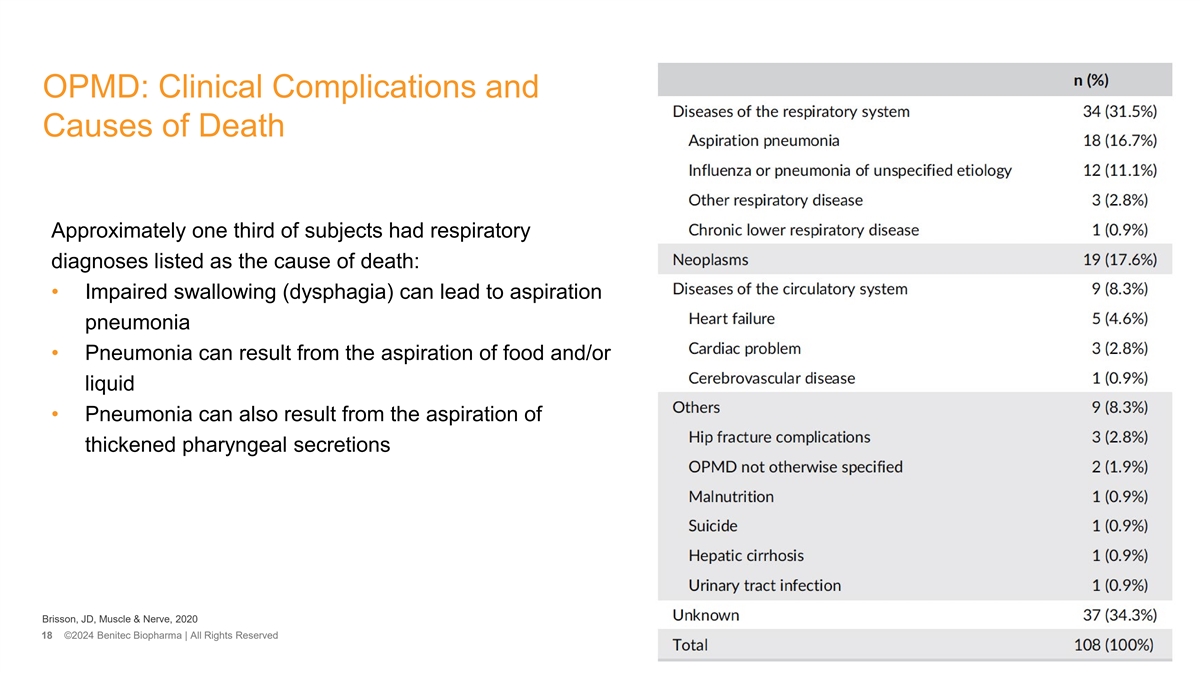
OPMD: Clinical Complications and Causes of Death Approximately one third of subjects had respiratory diagnoses listed as the cause of death: • Impaired swallowing (dysphagia) can lead to aspiration pneumonia • Pneumonia can result from the aspiration of food and/or liquid • Pneumonia can also result from the aspiration of thickened pharyngeal secretions Brisson, JD, Muscle & Nerve, 2020 18 ©2024 Benitec Biopharma | All Rights Reserved

Clinical Management of Dysphagia • Nutritional recommendations including adaptations • Surgical Interventions for moderate to severe dysphagia: – Cricopharyngeal muscle paralysis with botulinum toxin injection (temporary effect in some patients, requires repeated administration) – Cricopharyngeal muscle dilation (temporary effect, requires repeated application) – Cricopharyngeal myotomy (clinical/subject-reported outcomes suggest temporary delay of disease progression) • In all cases the pharyngeal constrictor muscles continue to atrophy, leading to progressive loss of pharyngeal propulsion/clearance of food and liquid into the esophagus 19 ©2024 B enitec B iopharm a | All R ights R es erved

BB-301: Mechanism of Action and Clinical Development Program Jerel A. Banks, MD, PhD 20 ©2024 Benitec Biopharma | All Rights Reserved

BB-301 Construct Design and Mechanism of Action 21 ©2024 Benitec Biopharma | All Rights Reserved
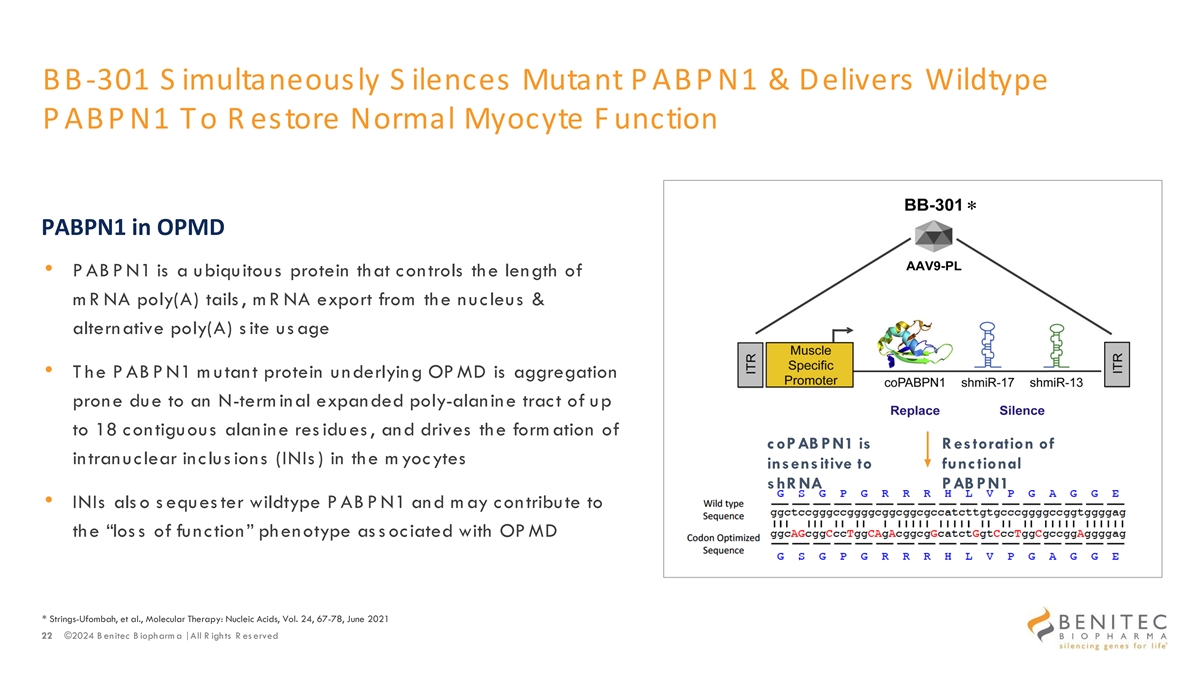
BB -301 S imultaneously S ilences Mutant P ABP N1 & Delivers Wildtype P ABP N1 To R estore Normal Myocyte F unction Design of L ead C andidate BB-301 * PABPN1 in OPMD • P AB P N1 is a ubiquitous protein that controls the length of m R NA poly(A) tails , m R NA export from the nucleus & alternative poly(A) s ite us age • T he P AB P N1 m utant protein underlying OP MD is aggregation prone due to an N-term inal expanded poly-alanine tract of up to 18 contiguous alanine res idues , and drives the form ation of coPAB PN1 is R estoration of intranuclear inclus ions (INIs ) in the m yocytes insensitive to func tional shR NA PAB PN1 • INIs als o s eques ter wildtype P AB P N1 and m ay contribute to the “los s of function” phenotype as s ociated with OP MD * Strings-Ufombah, et al., Molecular Therapy: Nucleic Acids, Vol. 24, 67-78, June 2021 22 ©2024 B enitec B iopharm a | All R ights R es erved
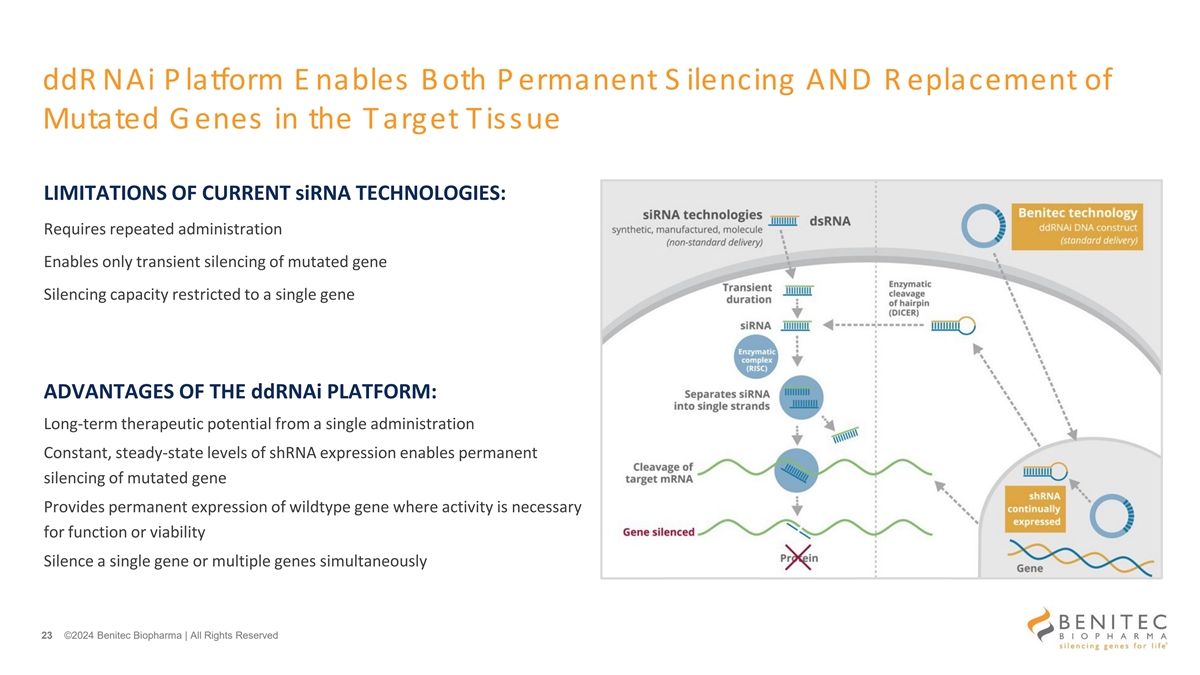
ddR NAi P latform E nables Both P ermanent S ilencing AND R eplacement of Mutated G enes in the Target Tissue LIMITATIONS OF CURRENT siRNA TECHNOLOGIES: Requires repeated administration Enables only transient silencing of mutated gene Silencing capacity restricted to a single gene ADVANTAGES OF THE ddRNAi PLATFORM: Long-term therapeutic potential from a single administration Constant, steady-state levels of shRNA expression enables permanent silencing of mutated gene Provides permanent expression of wildtype gene where activity is necessary for function or viability Silence a single gene or multiple genes simultaneously 23 ©2024 Benitec Biopharma | All Rights Reserved
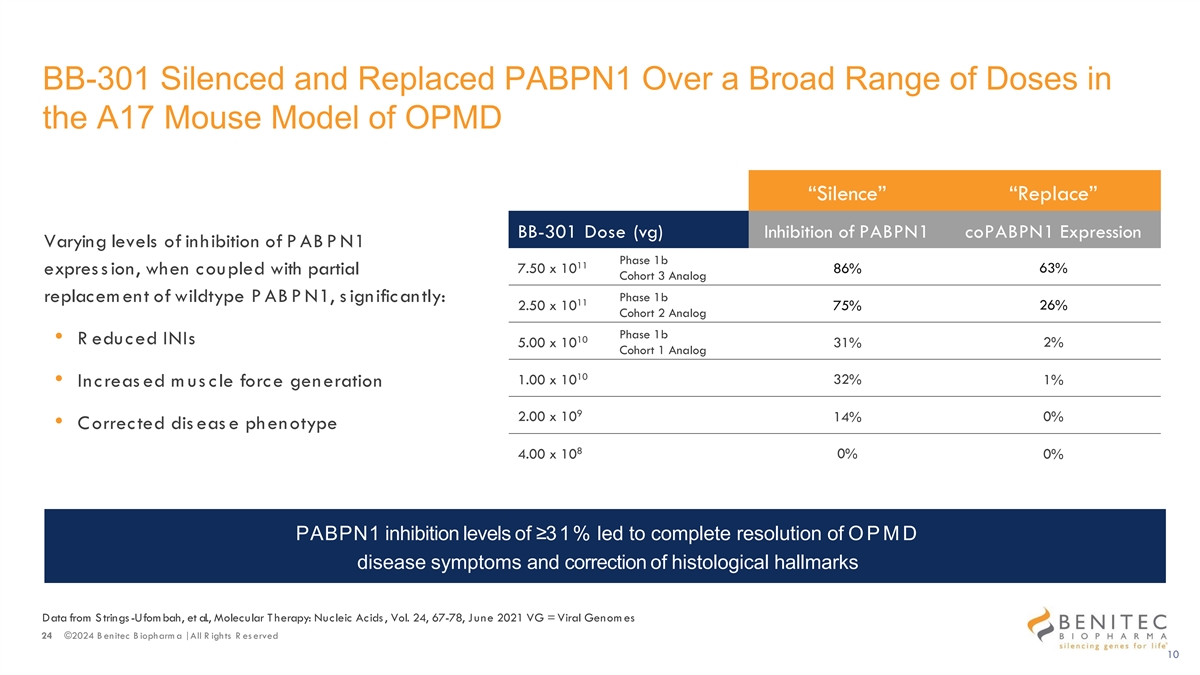
BB-301 Silenced and Replaced PABPN1 Over a Broad Range of Doses in the A17 Mouse Model of OPMD “Silence” “Replace” BB-301 Dose (vg) Inhibition of PABPN1 coPABPN1 Expression Varying levels of inhibition of PABPN1 Phase 1b 11 63% 7.50 x 10 86% expres s ion, when coupled with partial Cohort 3 Analog Phase 1b replacem ent of wildtype PABPN1, s ignificantly: 11 2.50 x 10 75% 26% Cohort 2 Analog Phase 1b 10 • R educed INIs 5.00 x 10 31% 2% Cohort 1 Analog 10 32% 1.00 x 10 1% • Increas ed m us cle force generation 9 2.00 x 10 14% 0% • Corrected dis eas e phenotype 8 4.00 x 10 0% 0% PABPN1 inhibition levels of ≥31% led to complete resolution of OP M D disease symptoms and correction of histological hallmarks Data from S trings-Ufom bah, et al., Molecular T herapy: Nucleic Acids , Vol. 24, 67-78, June 2021 VG = Viral Genom es 24 ©2024 B enitec B iopharm a | All R ights R es erved 10
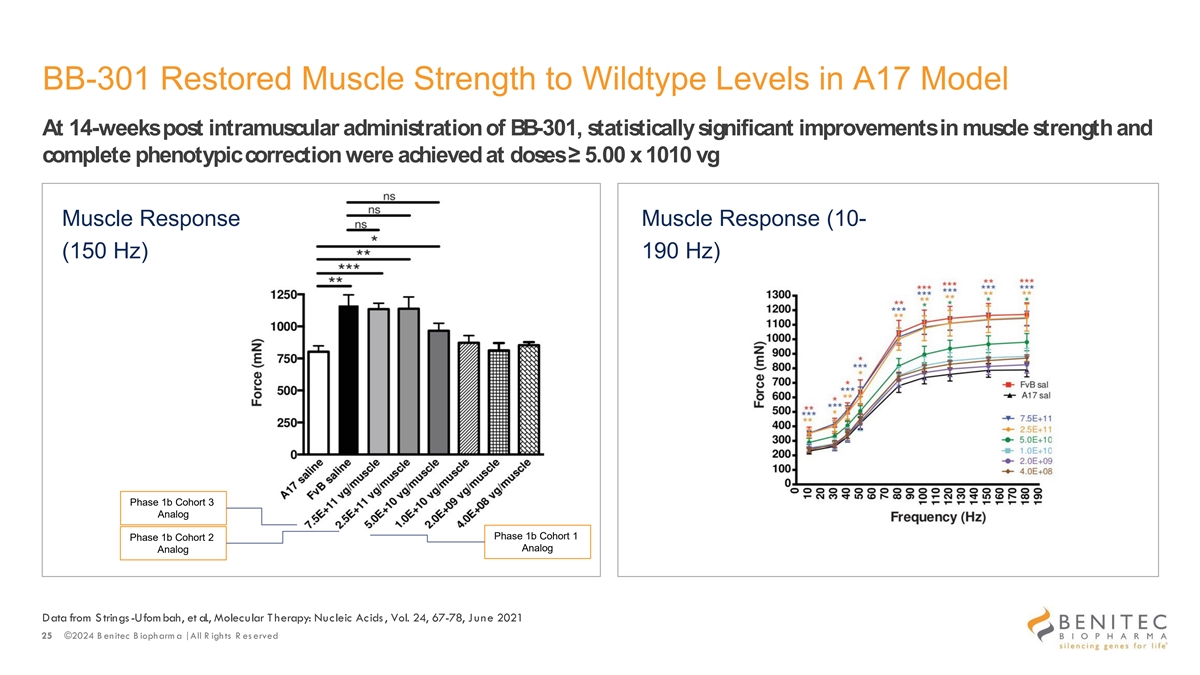
BB-301 Restored Muscle Strength to Wildtype Levels in A17 Model At 14-weeks post intramuscular administration of BB-301, statistically significant improvements in muscle strength and complete phenotypic correction were achieved at doses ≥ 5.00 x 1010 vg Muscle Response Muscle Response (10- (150 Hz) 190 Hz) Phase 1b Cohort 3 Analog Phase 1b Cohort 1 Phase 1b Cohort 2 Analog Analog Data from S trings-Ufom bah, et al., Molecular T herapy: Nucleic Acids , Vol. 24, 67-78, June 2021 25 ©2024 B enitec B iopharm a | All R ights R es erved
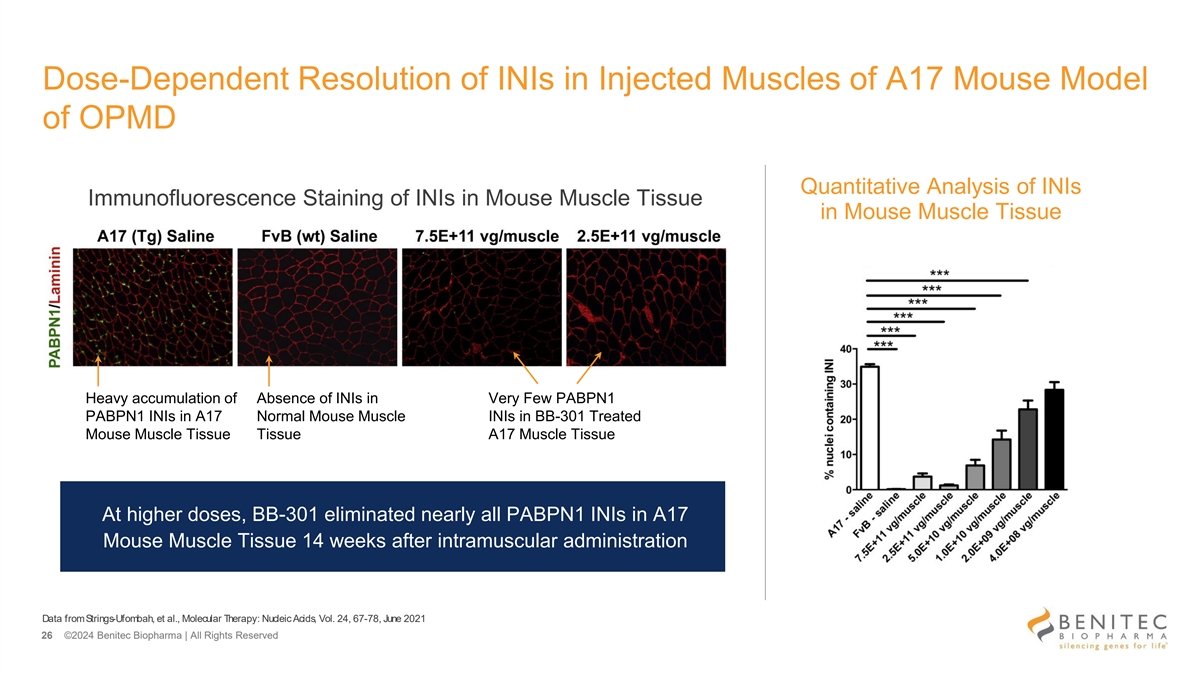
Dose-Dependent Resolution of INIs in Injected Muscles of A17 Mouse Model of OPMD Quantitative Analysis of INIs Immunofluorescence Staining of INIs in Mouse Muscle Tissue in Mouse Muscle Tissue Heavy accumulation of Absence of INIs in Very Few PABPN1 PABPN1 INIs in A17 Normal Mouse Muscle INIs in BB-301 Treated Mouse Muscle Tissue Tissue A17 Muscle Tissue At higher doses, BB-301 eliminated nearly all PABPN1 INIs in A17 Mouse Muscle Tissue 14 weeks after intramuscular administration Data from Strings-Ufombah, et al., Molecular Therapy: Nucleic Acids, Vol. 24, 67-78, June 2021 26 ©2024 Benitec Biopharma | All Rights Reserved

The Rationale for BB-301 in OPMD • In OPMD, the pharyngeal constrictor muscles are weakened and atrophic and, as a result, are unable to consistently exert the level of force required to support the propulsion of the food and liquid bolus • In the preclinical efficacy studies for BB-301 carried out in the A17 mouse model, direct intramuscular injection of BB-301 facilitated increases in muscle cross-sectional area, increases in muscle mass, and increases in muscle force generating capacity relative to untreated A17 mice • In the Beagle dog BB-301 dosing studies, intramuscular injections of BB-301 into the pharyngeal constrictor muscles supported dose- dependent tissue transduction, transgene expression, and target gene knockdown in the injected muscles • Restoration of muscle fiber size and muscle force generating capacity in the weakened and atrophic pharyngeal constrictor muscles of OPMD patients following BB-301 administration would be expected to meaningfully enhance the ability of the pharyngeal constrictor muscles to support food bolus propulsion through the pharynx and towards the esophagus, reducing dysphagia in OPMD patients 27 ©2024 B enitec B iopharm a | All R ights R es erved

BB-301 Clinical Development Program 28 ©2024 Benitec Biopharma | All Rights Reserved
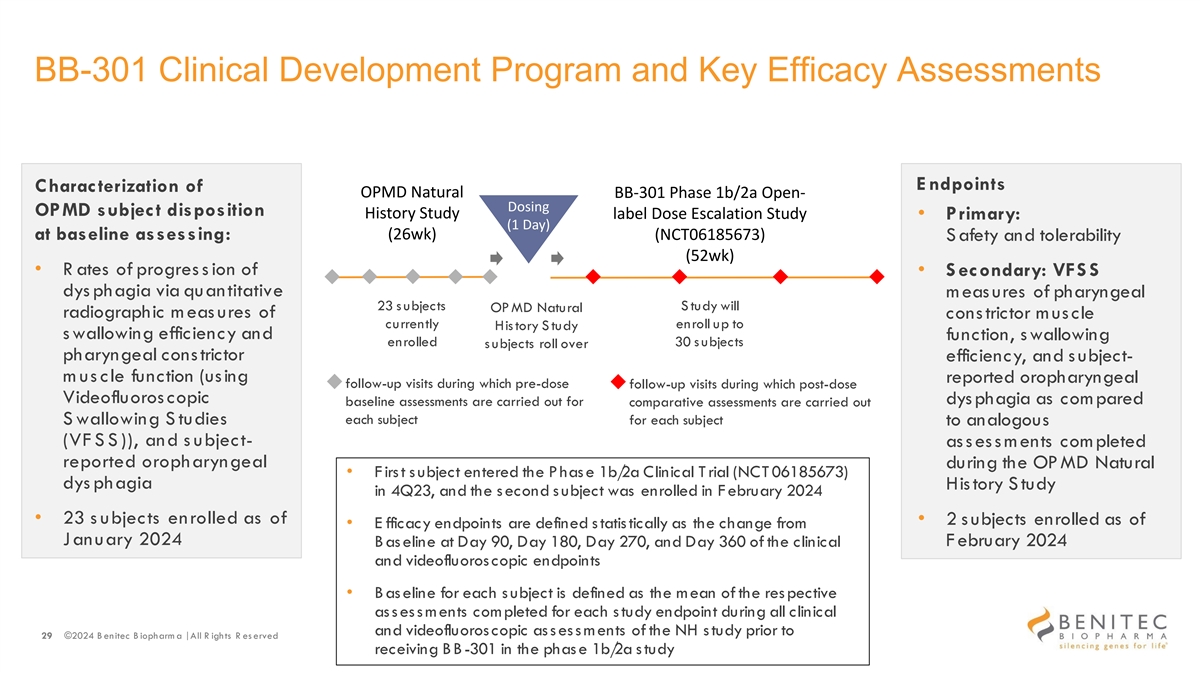
BB-301 Clinical Development Program and Key Efficacy Assessments E ndpoints Characterization of OPMD Natural BB-301 Phase 1b/2a Open- Dosing OPMD subject disposition History Study label Dose Escalation Study • Primary: (1 Day) at baseline assessing: (26wk) (NCT06185673) S afety and tolerability (52wk) • R ates of progres s ion of • S econdary: VFS S dys phagia via quantitative m eas ures of pharyngeal 23 s ubjects S tudy will OP MD Natural radiographic meas ures of constrictor muscle currently enroll up to His tory S tudy s wallowing efficiency and function, swallowing enrolled 30 s ubjects s ubjects roll over pharyngeal constrictor efficiency, and s ubject- m us cle function (using reported oropharyngeal follow-up visits during which pre-dose follow-up visits during which post-dose Videofluoros copic dys phagia as com pared baseline assessments are carried out for comparative assessments are carried out each subject S wallowing S tudies for each subject to analogous (VF S S )), and s ubject- assessments completed reported oropharyngeal during the OP MD Natural • F irs t s ubject entered the P has e 1b/ 2a Clinical T rial (NCT 06185673) dys phagia History Study in 4Q23, and the s econd s ubject was enrolled in F ebruary 2024 • 23 s ubjects enrolled as of • 2 s ubjects enrolled as of • E fficacy endpoints are defined s tatis tically as the change from January 2024 February 2024 B as eline at Day 90, Day 180, Day 270, and Day 360 of the clinical and videofluoroscopic endpoints • B as eline for each s ubject is defined as the mean of the res pective as s es s ments completed for each s tudy endpoint during all clinical and videofluoroscopic as s es s m ents of the NH s tudy prior to 29 ©2024 B enitec B iopharm a | All R ights R es erved receiving BB-301 in the phas e 1b/ 2a s tudy

Outcome Measures for the OPMD Natural History Study and the BB-301 Phase 1b/2a Clinical Study (NCT06185673) Videofluoroscopic Swallowing Study Assessments Clinical Assessments Global Swallowing Pharyngeal Constrictor Swallowing Efficiency Other Assessments Function Muscle Function Total Pharyngeal Residue Clinical measures of Dynamic Imaging Grade swallowing capacity & Pharyngeal Area at Vallecular Residue of Swallowing Toxicity dysphagia (including Maximum Constriction Scale timed-based and Pyriform Sinus Residue Pharyngeal Constriction volume-based drinking Ratio Other Pharyngeal tests) Residue Patient-reported Normalized Residue measures of dysphagia Ratio Scale 30 ©2024 Benitec Biopharma | All Rights Reserved

Videofluoroscopic Swallowing Studies and Subject-Reported Outcome Measures: Clinical and Methodological Overview Emily Plowman, PhD, CCC-SLP, FASHA 31 ©2024 Benitec Biopharma | All Rights Reserved

K ey Videofluoroscopic and S ubject-R eported E ndpoints The primary and secondary outcome measures for the OPMD Natural History Study and the BB-301 Phase 1b/2a Clinical Study (NCT06185673) facilitate serial characterization of: • Pharyngeal Constrictor Muscle Function • Swallowing Efficiency • Subject-Reported Oropharyngeal Dysphagia Swallowing tasks employed during the conduct of the VFSS are effort independent Videofluoroscopic swallowing studies are employed to complete several of the analyses outlined above, and the imaging results are reviewed and rated via a standardized, blinded process • Blinded central review of the respective fluoroscopic images by multiple independent Speech Language Pathologists is used for all assessments • Individual reviewers are assigned fluoroscopic studies to review and rate in a blinded manner (i.e., no knowledge of the other rater’s scores, subject ID, task, consistency, volume, time point) • The ratings are completed in full, and discrepancies are resolved during a consensus meeting 32 ©2024 B enitec B iopharm a | All R ights R es erved
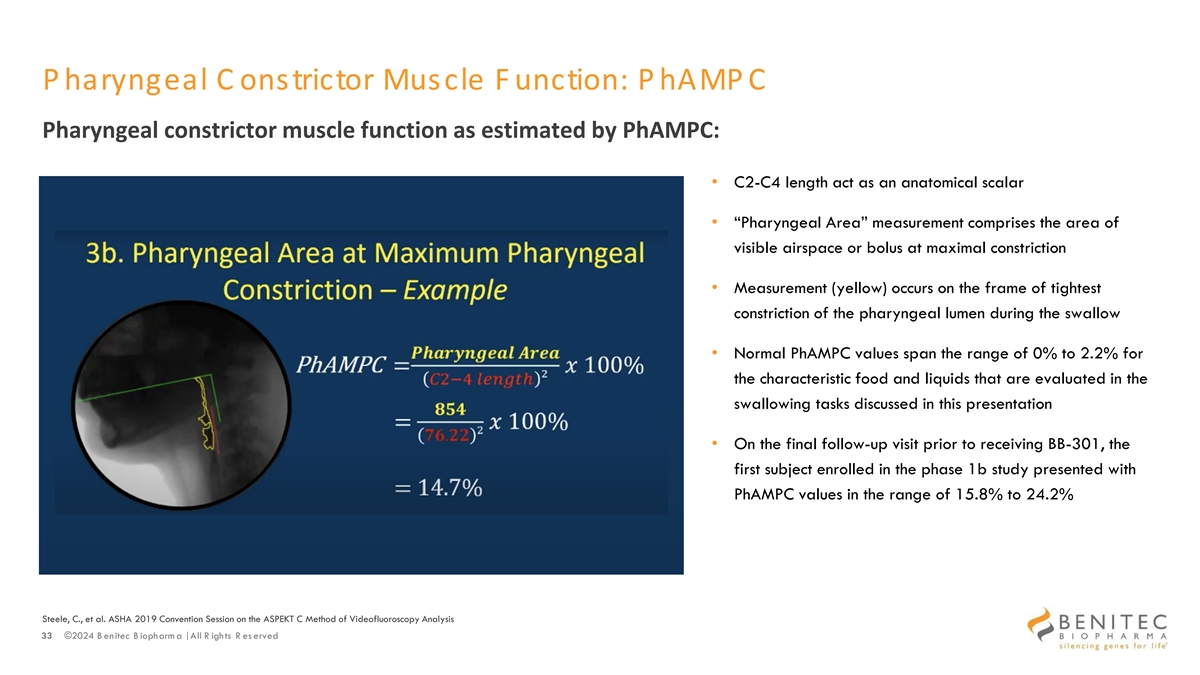
P haryngeal C onstrictor Muscle F unction: P hAMP C Pharyngeal constrictor muscle function as estimated by PhAMPC: • C2-C4 length act as an anatomical scalar • “Pharyngeal Area” measurement comprises the area of visible airspace or bolus at maximal constriction • Measurement (yellow) occurs on the frame of tightest constriction of the pharyngeal lumen during the swallow • Normal PhAMPC values span the range of 0% to 2.2% for the characteristic food and liquids that are evaluated in the swallowing tasks discussed in this presentation • On the final follow-up visit prior to receiving BB-301, the first subject enrolled in the phase 1b study presented with PhAMPC values in the range of 15.8% to 24.2% Steele, C., et al. ASHA 2019 Convention Session on the ASPEKT C Method of Videofluoroscopy Analysis 33 ©2024 B enitec B iopharm a | All R ights R es erved
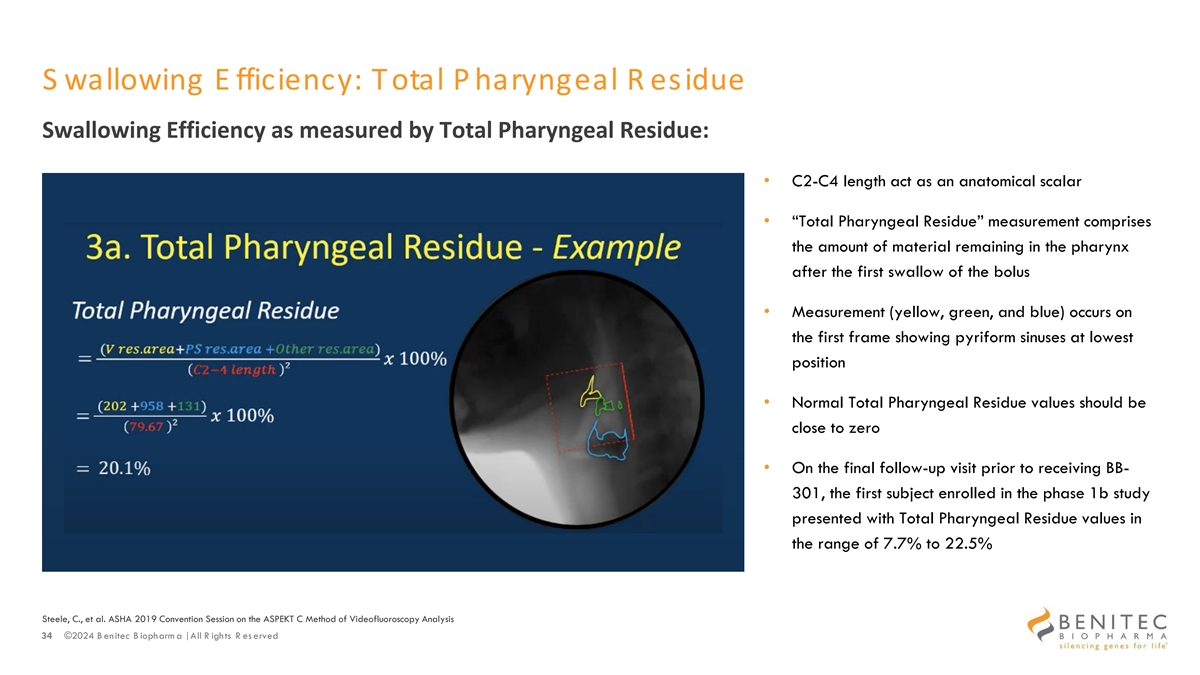
S wallowing E fficiency: Total P haryngeal R esidue Swallowing Efficiency as measured by Total Pharyngeal Residue: • C2-C4 length act as an anatomical scalar • “Total Pharyngeal Residue” measurement comprises the amount of material remaining in the pharynx after the first swallow of the bolus • Measurement (yellow, green, and blue) occurs on the first frame showing pyriform sinuses at lowest position • Normal Total Pharyngeal Residue values should be close to zero • On the final follow-up visit prior to receiving BB- 301, the first subject enrolled in the phase 1b study presented with Total Pharyngeal Residue values in the range of 7.7% to 22.5% Steele, C., et al. ASHA 2019 Convention Session on the ASPEKT C Method of Videofluoroscopy Analysis 34 ©2024 B enitec B iopharm a | All R ights R es erved
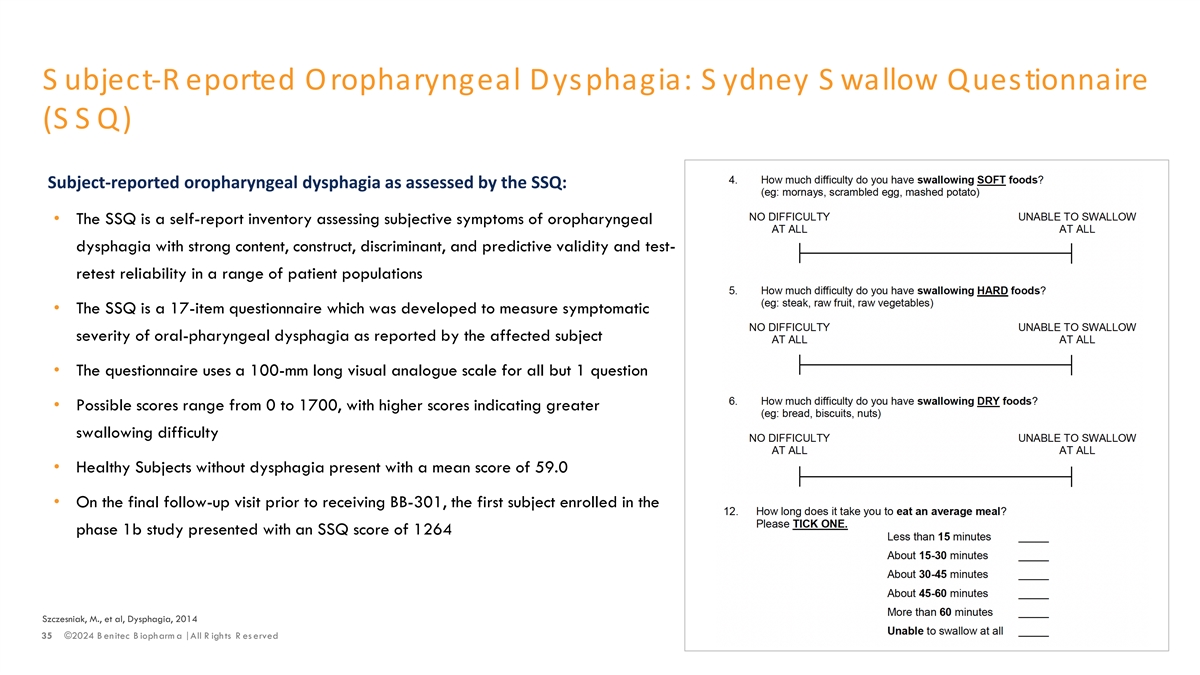
S ubject-R eported Oropharyngeal Dysphagia: S ydney S wallow Questionnaire (SSQ) Subject-reported oropharyngeal dysphagia as assessed by the SSQ: • The SSQ is a self-report inventory assessing subjective symptoms of oropharyngeal dysphagia with strong content, construct, discriminant, and predictive validity and test- retest reliability in a range of patient populations • The SSQ is a 17-item questionnaire which was developed to measure symptomatic severity of oral-pharyngeal dysphagia as reported by the affected subject • The questionnaire uses a 100-mm long visual analogue scale for all but 1 question • Possible scores range from 0 to 1700, with higher scores indicating greater swallowing difficulty • Healthy Subjects without dysphagia present with a mean score of 59.0 • On the final follow-up visit prior to receiving BB-301, the first subject enrolled in the phase 1b study presented with an SSQ score of 1264 Szczesniak, M., et al, Dysphagia, 2014 35 ©2024 B enitec B iopharm a | All R ights R es erved

Clinically Meaningful Improvements Clinically meaningful improvement over the course of the BB-301 clinical development program will be defined by: • Improvements in Subject-Reported Outcome assessments (i.e., reductions in the Sydney Swallow Questionnaire [”SSQ”] Scores) post BB-301 dose and Reductions in Total Pharyngeal Residue (i.e., reductions in the total food or liquid material remaining in the pharynx at the completion of swallowing) post BB-301 dose Specific attention will be given to the following: • Improvements in Subject-Reported Outcome assessments (i.e., SSQ Scores) post BB-301 dose that are accompanied by similar improvements in videofluoroscopic swallowing study assessments (i.e., reductions in PhAMPC% and/or reductions in Total Pharyngeal Residue across one or more consistencies of liquid and/or solid food) • Improvements in the results of individual outcome measures post BB-301 dose as compared to the results of the analogous assessments conducted at Visit 1 of the OPMD Natural History Study (i.e., 6 to 12 months prior to the receipt of BB-301) 36 ©2024 B enitec B iopharm a | All R ights R es erved

Preliminary BB-301 Phase 1b Clinical Data Summary for the First Subject (Day 90) Jerel A. Banks, MD, PhD 37 ©2024 Benitec Biopharma | All Rights Reserved

Key Questions for Subject 1 of the Phase 1b Study Subject 1 experienced disease progression during their enrollment in the Natural History Study In this regard, several critical questions emerged with respect to the potential impact of BB-301: • Would BB-301 slow progression, halt, or improve dysphagia in this study subject? • Would the current, low dose of BB-301 be sufficiently biologically active to facilitate a benefit in this study subject? Would this benefit be visible at the first follow-up assessments conducted at Day 90 post-dose? • Would BB-301 cause any Serious Adverse Events? 38 ©2024 Benitec Biopharma | All Rights Reserved

VFSS: PhAMPC Results 39 ©2024 Benitec Biopharma | All Rights Reserved
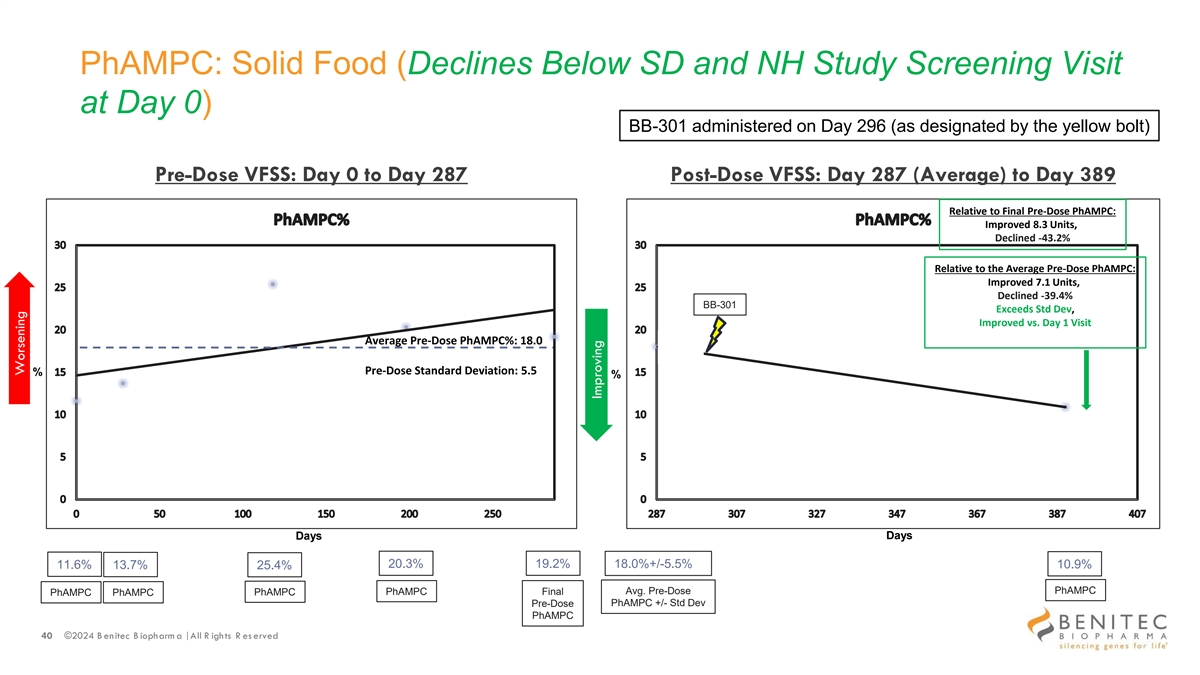
PhAMPC: Solid Food (Declines Below SD and NH Study Screening Visit at Day 0) BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose PhAMPC: Improved 8.3 Units, Declined -43.2% Relative to the Average Pre-Dose PhAMPC: Improved 7.1 Units, Declined -39.4% BB-301 Exceeds Std Dev, Improved vs. Day 1 Visit Average Pre-Dose PhAMPC%: 18.0 Pre-Dose Standard Deviation: 5.5 % % Days Days 20.3% 19.2% 11.6% 13.7% 18.0%+/-5.5% 10.9% 25.4% PhAMPC PhAMPC Final Avg. Pre-Dose PhAMPC PhAMPC PhAMPC PhAMPC +/- Std Dev Pre-Dose PhAMPC 40 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
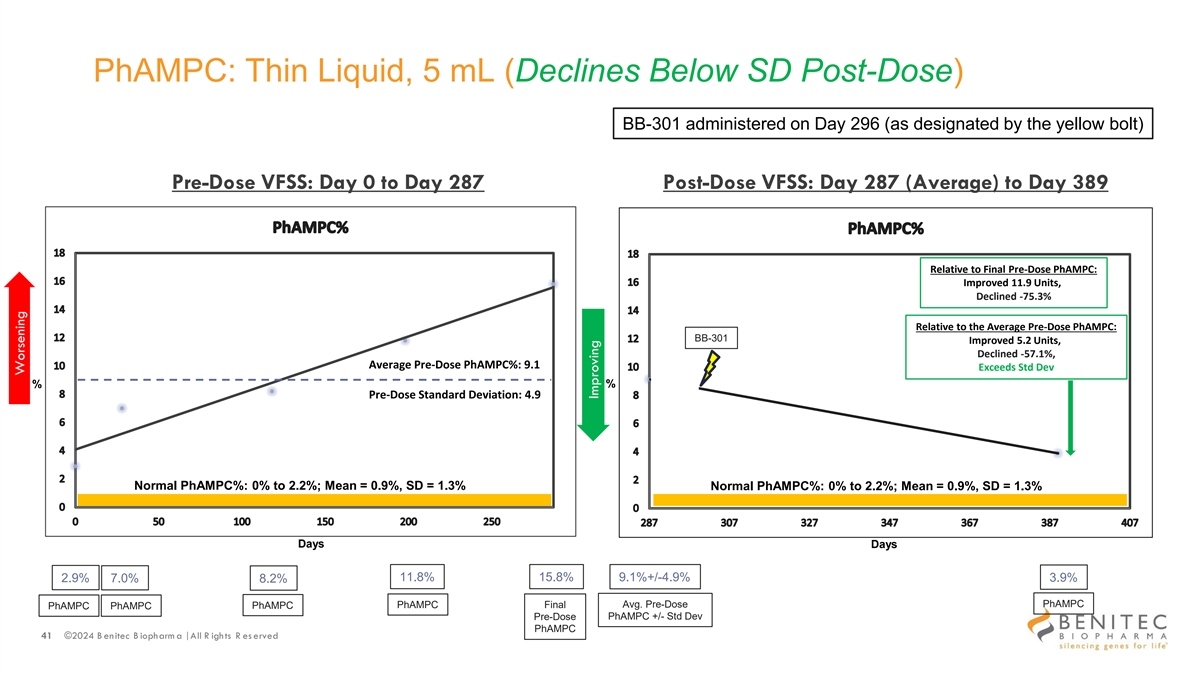
PhAMPC: Thin Liquid, 5 mL (Declines Below SD Post-Dose) BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose PhAMPC: Improved 11.9 Units, Declined -75.3% Relative to the Average Pre-Dose PhAMPC: BB-301 Improved 5.2 Units, Declined -57.1%, Average Pre-Dose PhAMPC%: 9.1 Exceeds Std Dev % % Pre-Dose Standard Deviation: 4.9 Normal PhAMPC%: 0% to 2.2%; Mean = 0.9%, SD = 1.3% Normal PhAMPC%: 0% to 2.2%; Mean = 0.9%, SD = 1.3% Days Days 11.8% 15.8% 9.1%+/-4.9% 2.9% 7.0% 8.2% 3.9% Avg. Pre-Dose PhAMPC PhAMPC PhAMPC Final PhAMPC PhAMPC Pre-Dose PhAMPC +/- Std Dev PhAMPC 41 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
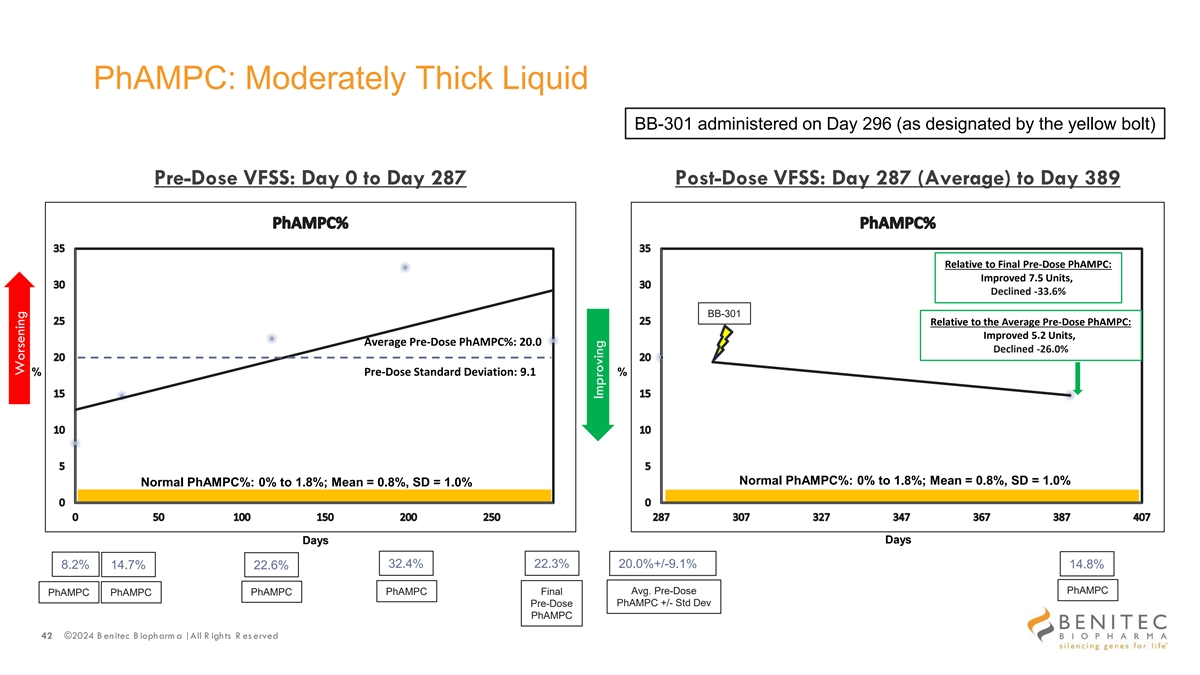
PhAMPC: Moderately Thick Liquid BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose PhAMPC: Improved 7.5 Units, Declined -33.6% BB-301 Relative to the Average Pre-Dose PhAMPC: Improved 5.2 Units, Average Pre-Dose PhAMPC%: 20.0 Declined -26.0% % Pre-Dose Standard Deviation: 9.1 % Normal PhAMPC%: 0% to 1.8%; Mean = 0.8%, SD = 1.0% Normal PhAMPC%: 0% to 1.8%; Mean = 0.8%, SD = 1.0% Days Days 32.4% 22.3% 8.2% 14.7% 20.0%+/-9.1% 14.8% 22.6% PhAMPC PhAMPC Final Avg. Pre-Dose PhAMPC PhAMPC PhAMPC PhAMPC +/- Std Dev Pre-Dose PhAMPC 42 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
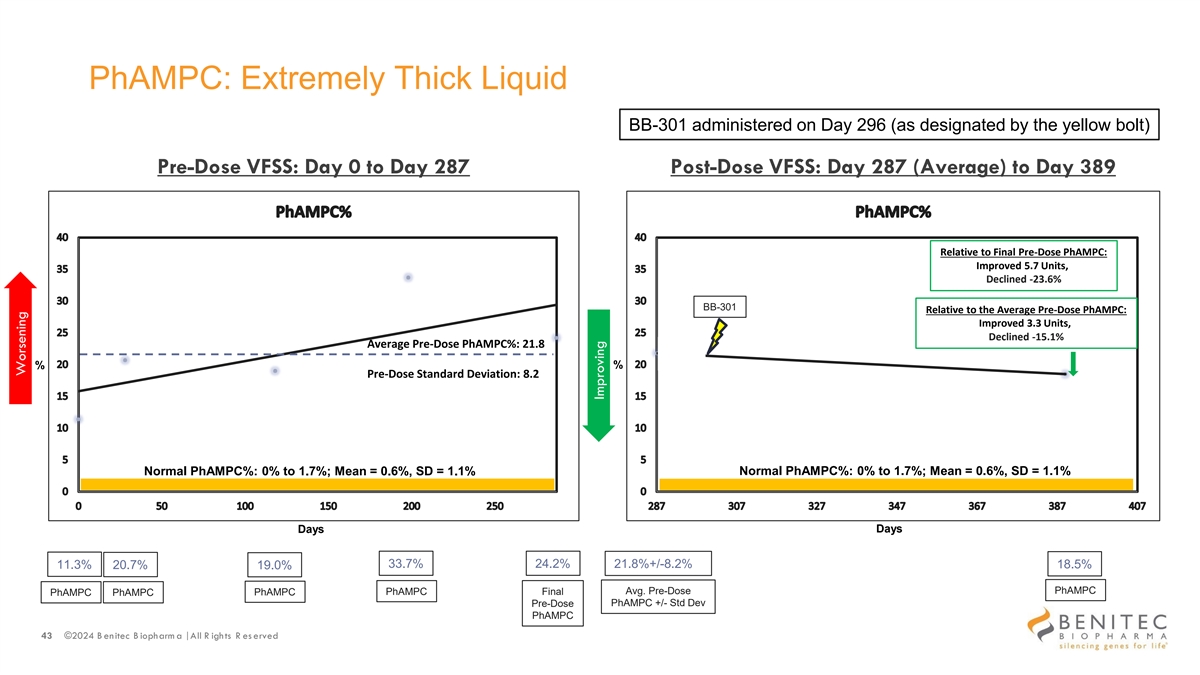
PhAMPC: Extremely Thick Liquid BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose PhAMPC: Improved 5.7 Units, Declined -23.6% BB-301 Relative to the Average Pre-Dose PhAMPC: Improved 3.3 Units, Declined -15.1% Average Pre-Dose PhAMPC%: 21.8 % % Pre-Dose Standard Deviation: 8.2 Normal PhAMPC%: 0% to 1.7%; Mean = 0.6%, SD = 1.1% Normal PhAMPC%: 0% to 1.7%; Mean = 0.6%, SD = 1.1% Days Days 33.7% 24.2% 11.3% 20.7% 21.8%+/-8.2% 18.5% 19.0% PhAMPC PhAMPC Final Avg. Pre-Dose PhAMPC PhAMPC PhAMPC PhAMPC +/- Std Dev Pre-Dose PhAMPC 43 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
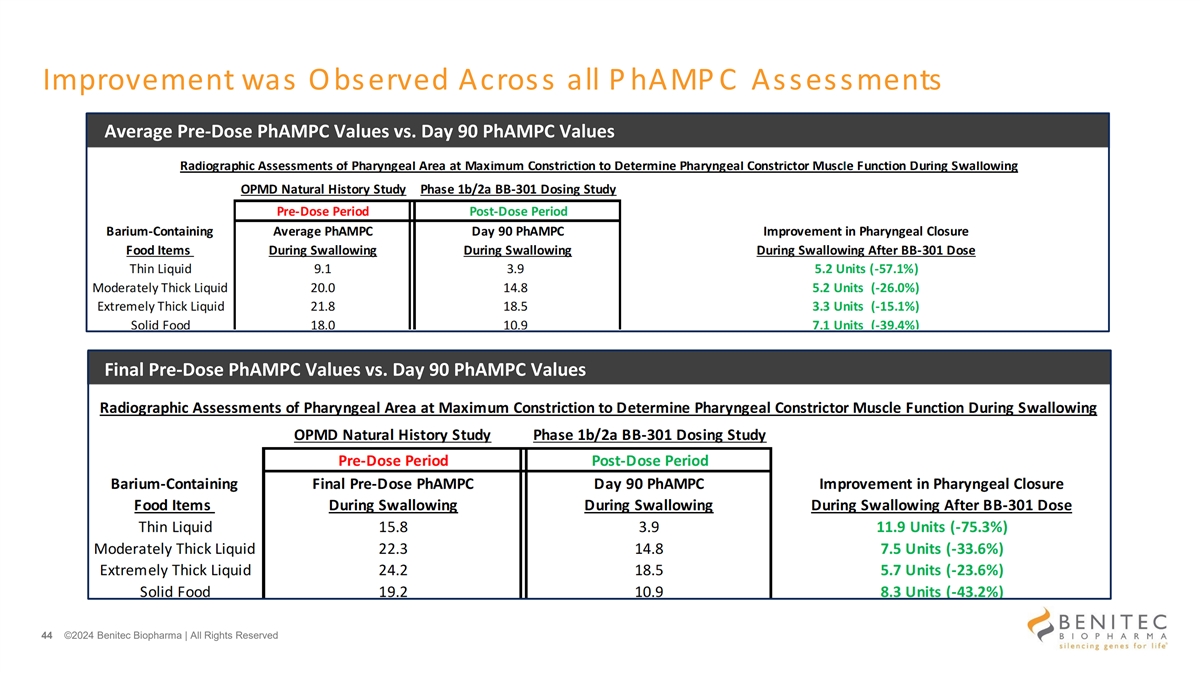
Improvement was Observed Across all P hAMP C Assessments Average Pre-Dose PhAMPC Values vs. Day 90 PhAMPC Values Final Pre-Dose PhAMPC Values vs. Day 90 PhAMPC Values 44 ©2024 Benitec Biopharma | All Rights Reserved

VFSS: Total Pharyngeal Residue Results 45 ©2024 Benitec Biopharma | All Rights Reserved
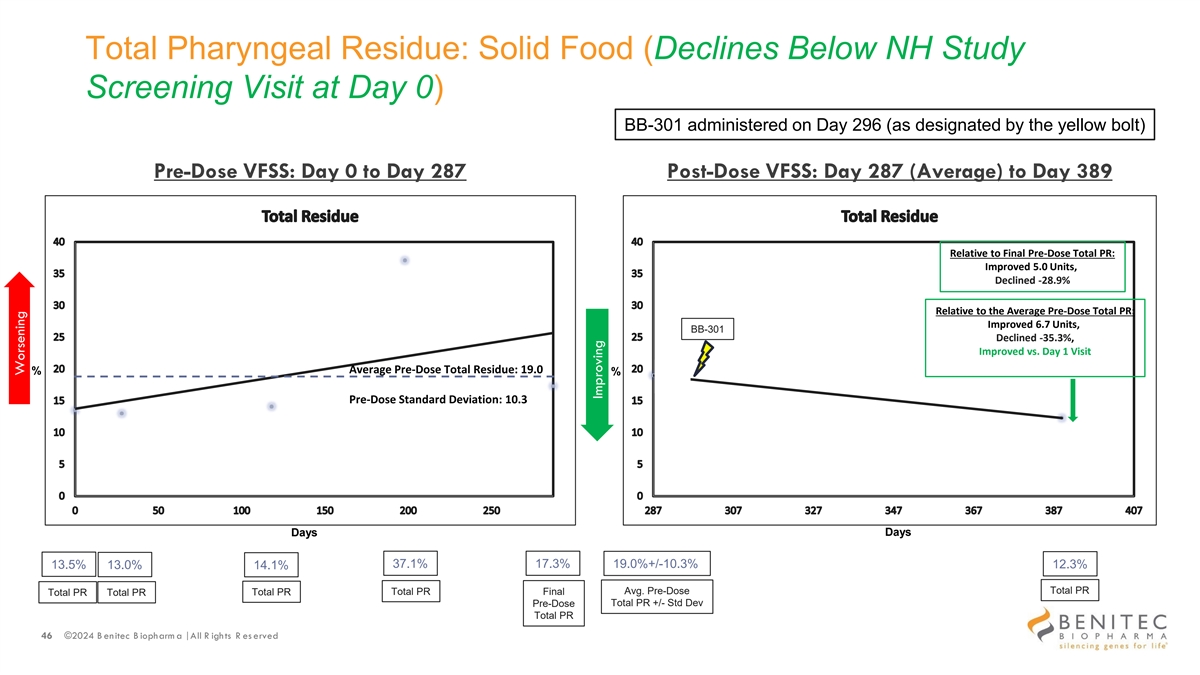
Total Pharyngeal Residue: Solid Food (Declines Below NH Study Screening Visit at Day 0) BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose Total PR: Improved 5.0 Units, Declined -28.9% Relative to the Average Pre-Dose Total PR: Improved 6.7 Units, BB-301 Declined -35.3%, Improved vs. Day 1 Visit Average Pre-Dose Total Residue: 19.0 % % Pre-Dose Standard Deviation: 10.3 Days Days 37.1% 17.3% 13.5% 13.0% 19.0%+/-10.3% 12.3% 14.1% Total PR Total PR Final Avg. Pre-Dose Total PR Total PR Total PR Total PR +/- Std Dev Pre-Dose Total PR 46 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
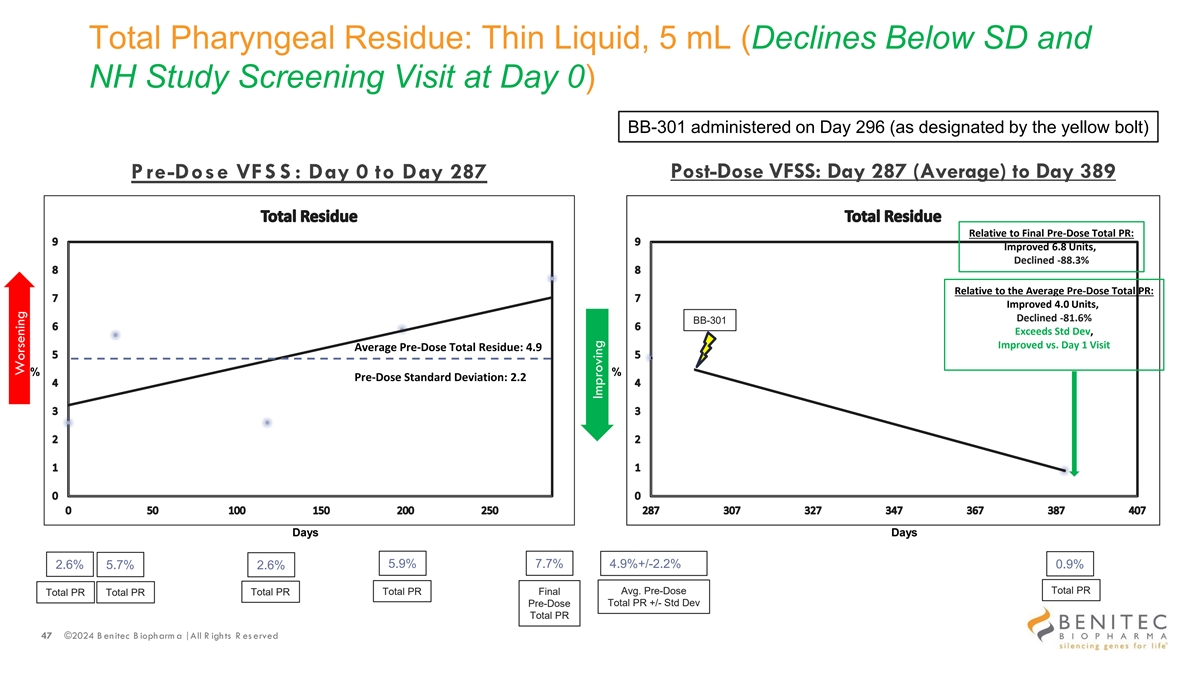
Total Pharyngeal Residue: Thin Liquid, 5 mL (Declines Below SD and NH Study Screening Visit at Day 0) BB-301 administered on Day 296 (as designated by the yellow bolt) Post-Dose VFSS: Day 287 (Average) to Day 389 P re-Dose VFS S : Day 0 to Day 287 Relative to Final Pre-Dose Total PR: Improved 6.8 Units, Declined -88.3% Relative to the Average Pre-Dose Total PR: Improved 4.0 Units, Declined -81.6% BB-301 Exceeds Std Dev, Improved vs. Day 1 Visit Average Pre-Dose Total Residue: 4.9 % % Pre-Dose Standard Deviation: 2.2 Days Days 5.9% 7.7% 2.6% 5.7% 4.9%+/-2.2% 0.9% 2.6% Total PR Total PR Final Avg. Pre-Dose Total PR Total PR Total PR Total PR +/- Std Dev Pre-Dose Total PR 47 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
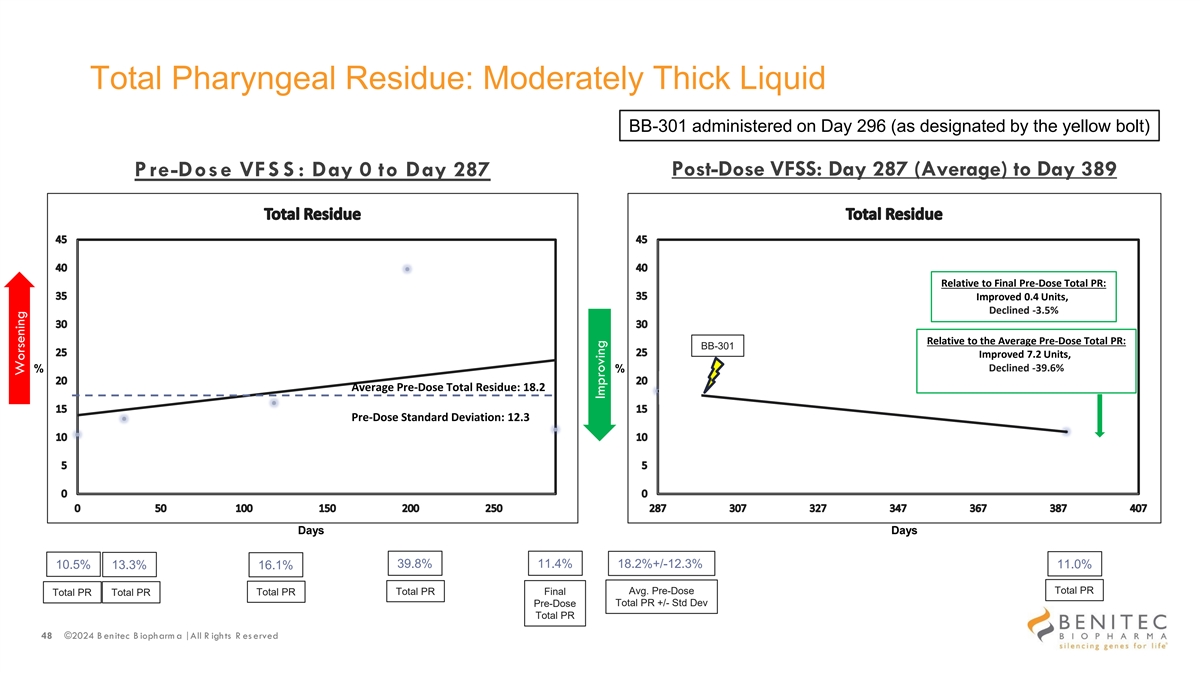
Total Pharyngeal Residue: Moderately Thick Liquid BB-301 administered on Day 296 (as designated by the yellow bolt) P re-Dose VFS S : Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose Total PR: Improved 0.4 Units, Declined -3.5% Relative to the Average Pre-Dose Total PR: BB-301 Improved 7.2 Units, Declined -39.6% % % Average Pre-Dose Total Residue: 18.2 Pre-Dose Standard Deviation: 12.3 Days Days 39.8% 11.4% 10.5% 13.3% 18.2%+/-12.3% 11.0% 16.1% Total PR Total PR Final Avg. Pre-Dose Total PR Total PR Total PR Total PR +/- Std Dev Pre-Dose Total PR 48 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
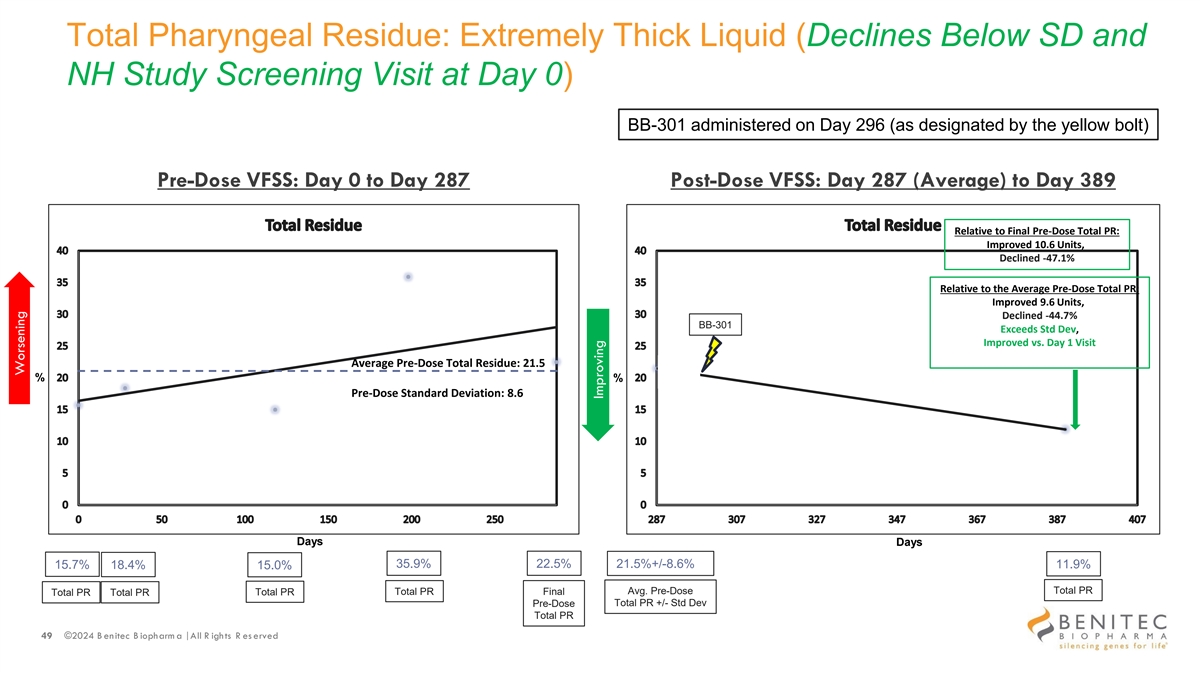
Total Pharyngeal Residue: Extremely Thick Liquid (Declines Below SD and NH Study Screening Visit at Day 0) BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose VFSS: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose Total PR: Improved 10.6 Units, Declined -47.1% Relative to the Average Pre-Dose Total PR: Improved 9.6 Units, Declined -44.7% BB-301 Exceeds Std Dev, Improved vs. Day 1 Visit Average Pre-Dose Total Residue: 21.5 % % Pre-Dose Standard Deviation: 8.6 Days Days 35.9% 22.5% 15.7% 18.4% 21.5%+/-8.6% 11.9% 15.0% Total PR Total PR Final Avg. Pre-Dose Total PR Total PR Total PR Total PR +/- Std Dev Pre-Dose Total PR 49 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Improving
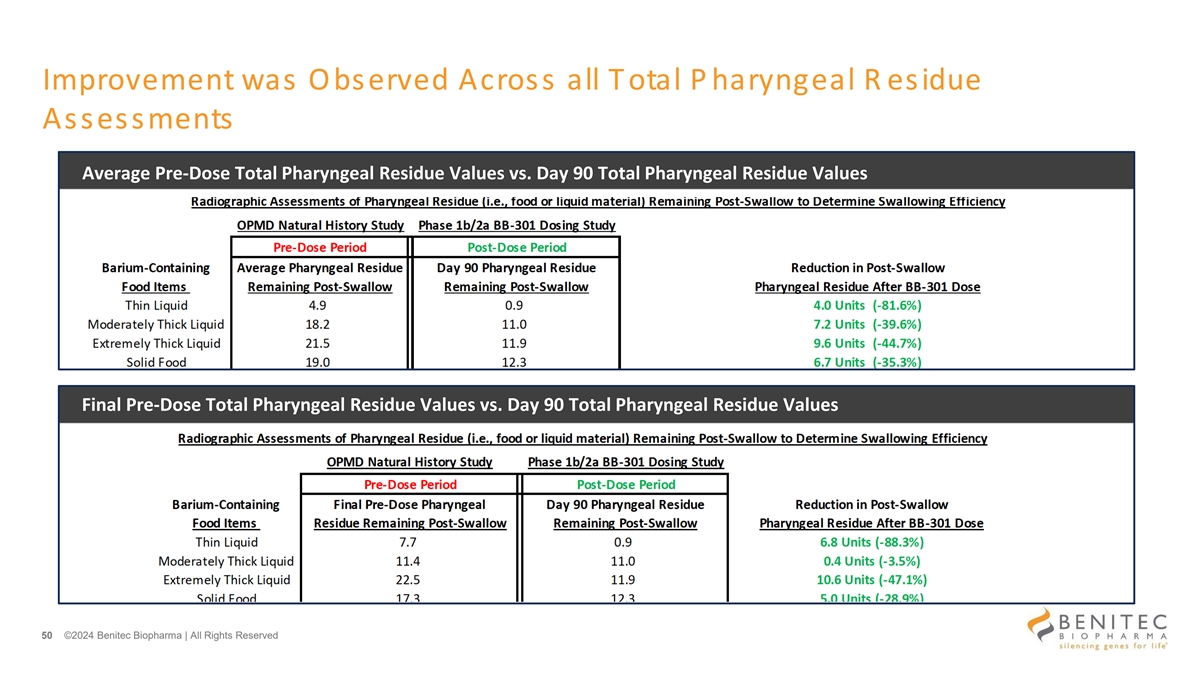
Improvement was Observed Across all Total P haryngeal R esidue Assessments Average Pre-Dose Total Pharyngeal Residue Values vs. Day 90 Total Pharyngeal Residue Values Final Pre-Dose Total Pharyngeal Residue Values vs. Day 90 Total Pharyngeal Residue Values 50 ©2024 Benitec Biopharma | All Rights Reserved

Cold Water Timed Drinking Test (CWDT) Results 51 ©2024 Benitec Biopharma | All Rights Reserved
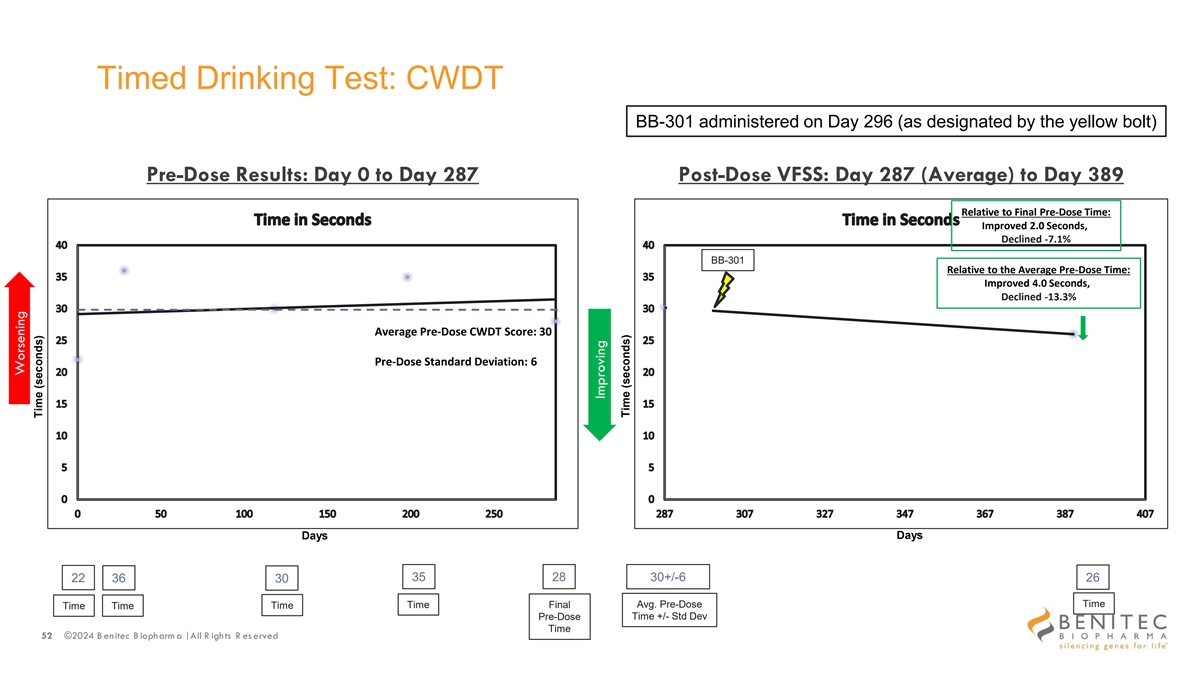
Timed Drinking Test: CWDT BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose Results: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose Time: Improved 2.0 Seconds, Declined -7.1% BB-301 Relative to the Average Pre-Dose Time: Improved 4.0 Seconds, Declined -13.3% Average Pre-Dose CWDT Score: 30 Pre-Dose Standard Deviation: 6 Days Days 35 28 30+/-6 22 36 30 26 Avg. Pre-Dose Time Time Time Final Time Time Pre-Dose Time +/- Std Dev Time 52 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Time (seconds) Improving Time (seconds)
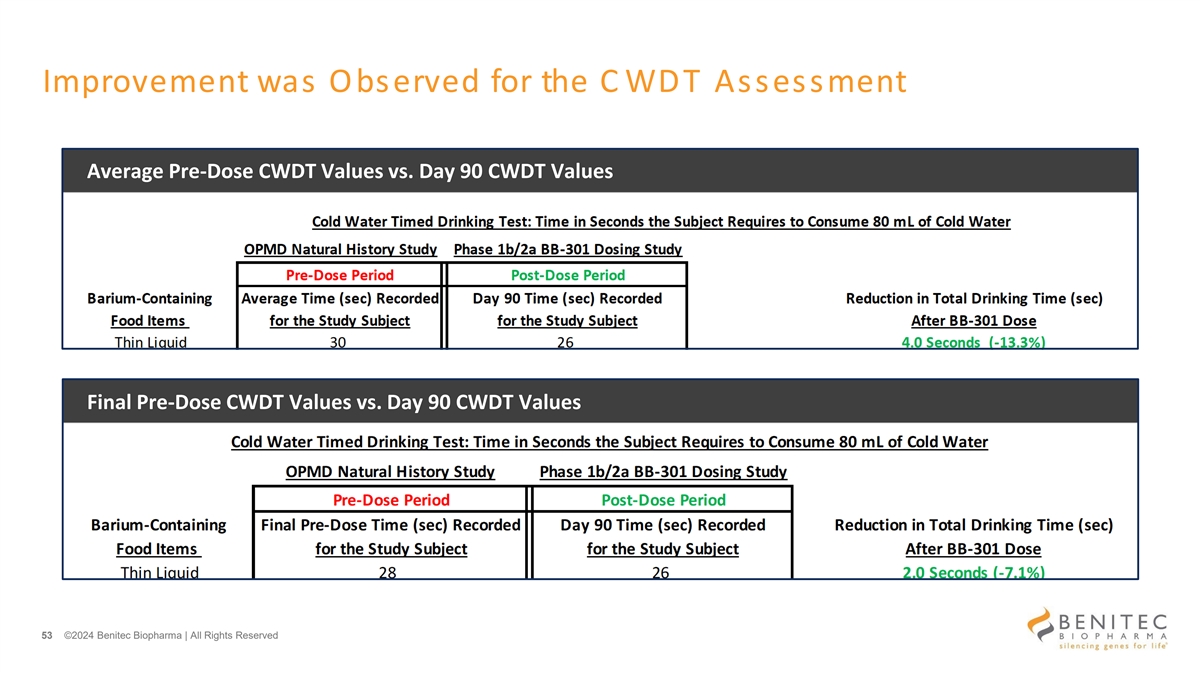
Improvement was Observed for the C WDT Assessment Average Pre-Dose CWDT Values vs. Day 90 CWDT Values Final Pre-Dose CWDT Values vs. Day 90 CWDT Values 53 ©2024 Benitec Biopharma | All Rights Reserved

SSQ Results 54 ©2024 Benitec Biopharma | All Rights Reserved
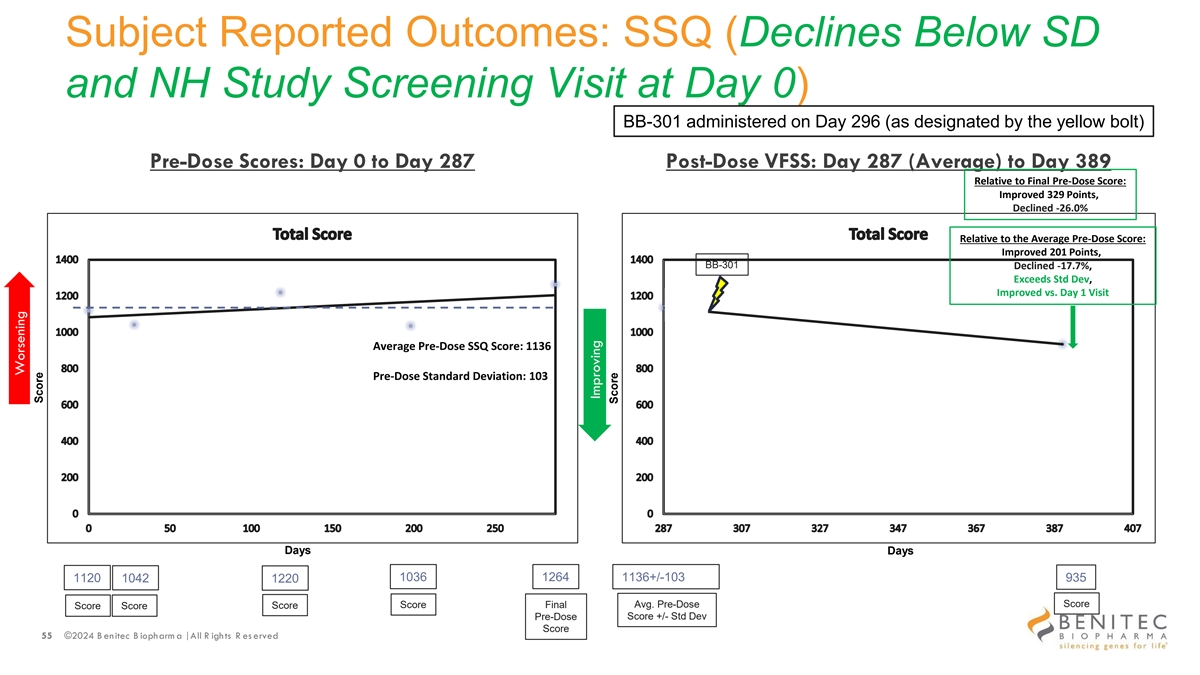
Subject Reported Outcomes: SSQ (Declines Below SD and NH Study Screening Visit at Day 0) BB-301 administered on Day 296 (as designated by the yellow bolt) Pre-Dose Scores: Day 0 to Day 287 Post-Dose VFSS: Day 287 (Average) to Day 389 Relative to Final Pre-Dose Score: Improved 329 Points, Declined -26.0% Relative to the Average Pre-Dose Score: Improved 201 Points, BB-301 Declined -17.7%, Exceeds Std Dev, Improved vs. Day 1 Visit Average Pre-Dose SSQ Score: 1136 Pre-Dose Standard Deviation: 103 Days Days 1036 1264 1136+/-103 1120 1042 1220 935 Avg. Pre-Dose Score Score Score Final Score Score Pre-Dose Score +/- Std Dev Score 55 ©2024 B enitec B iopharm a | All R ights R es erved Worsening Score Improving Score
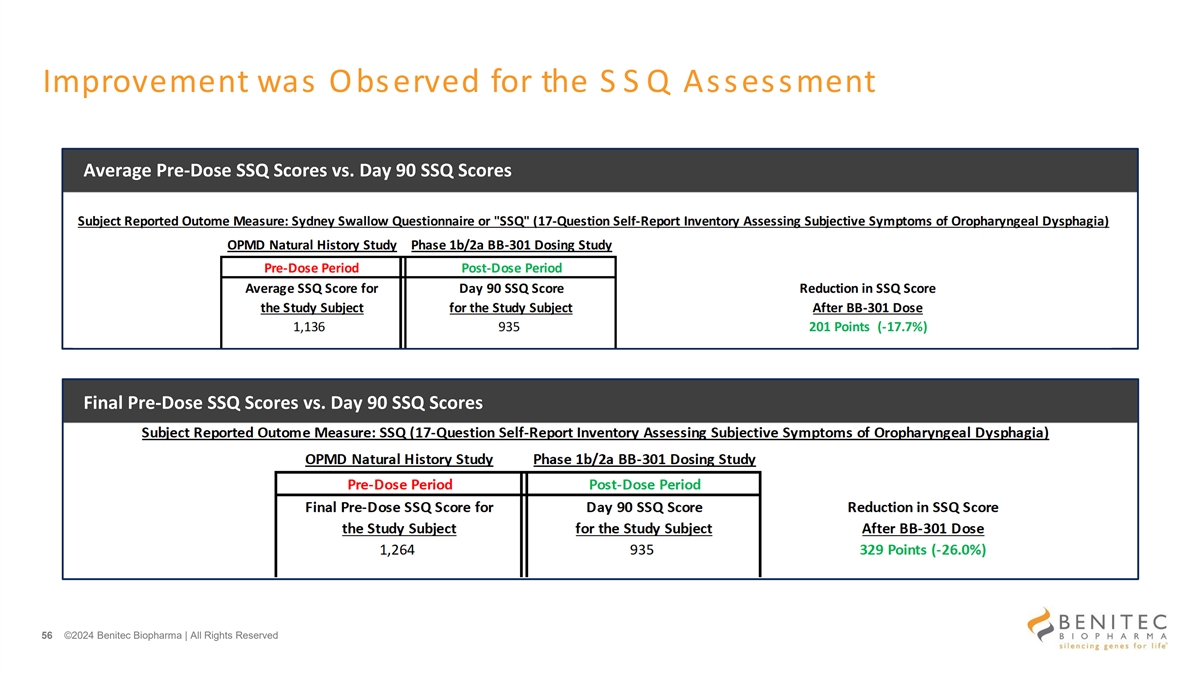
Improvement was Observed for the S S Q Assessment Average Pre-Dose SSQ Scores vs. Day 90 SSQ Scores Final Pre-Dose SSQ Scores vs. Day 90 SSQ Scores 56 ©2024 Benitec Biopharma | All Rights Reserved

C linically Meaningful Improvements Clinically meaningful improvement over the course of the BB-301 clinical development program will be defined by: • Improvements in Subject-Reported Outcome assessments (i.e., reductions in the Sydney Swallow Questionnaire [”SSQ”] Scores) post BB-301 dose and Reductions in Total Pharyngeal Residue (i.e., reductions in the total food or liquid material remaining in the pharynx at the completion of swallowing) post BB-301 dose Specific attention will be given to the following: • Improvements in Subject-Reported Outcome assessments (i.e., SSQ Scores) post BB-301 dose that are accompanied by similar improvements in videofluoroscopic swallowing study assessments (i.e., reductions in PhAMPC% and/or reductions in Total Pharyngeal Residue across one or more consistencies of liquid and/or solid food) • Improvements in the results of individual outcome measures post BB-301 dose as compared to the results of the analogous assessments conducted at Visit 1 of the OPMD Natural History Study (i.e., 6 to 12 months prior to the receipt of BB-301) 57 ©2024 B enitec B iopharm a | All R ights R es erved

Our Learnings for Subject 1 of the Phase 1b Study Subject 1 experienced disease progression during their enrollment in the Natural History Study After dosing: • BB-301 slowed improved dysphagia in this study subject • The current, low dose of BB-301 was sufficiently biologically active to facilitate a benefit in this study subject and these benefits were visible at the first follow-up assessments conducted at Day 90 post-dose • BB-301 did not cause any Serious Adverse Events 58 ©2024 B enitec B iopharm a | All R ights R es erved
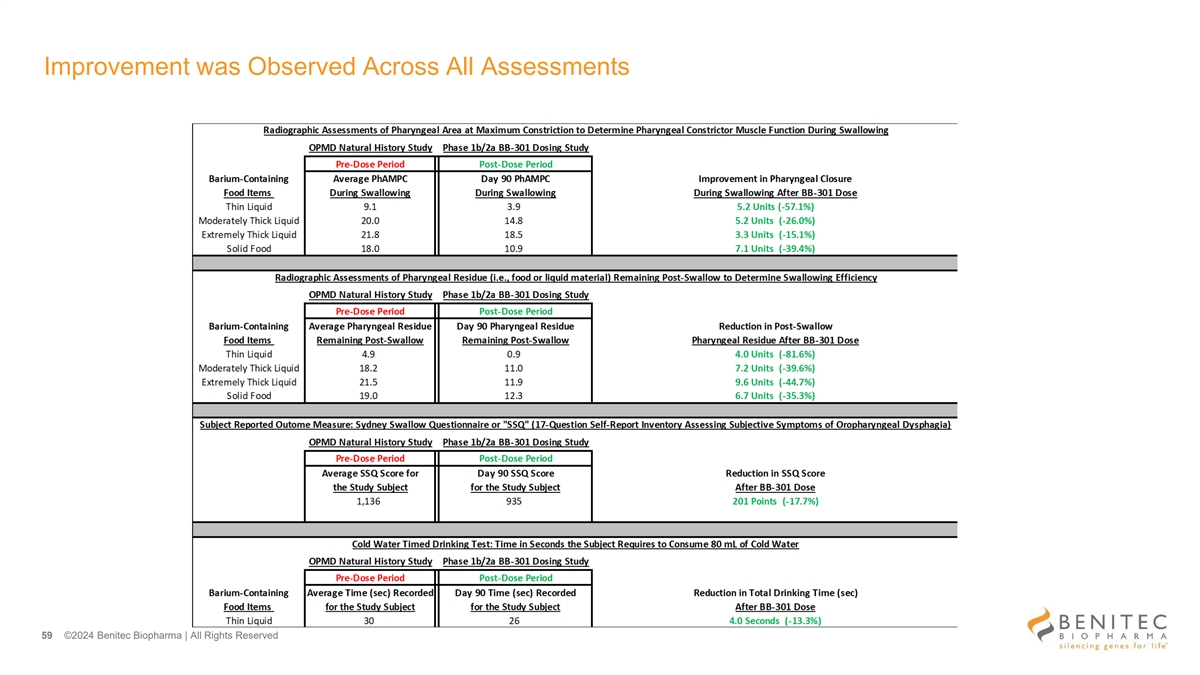
Improvement was Observed Across All Assessments 59 ©2024 Benitec Biopharma | All Rights Reserved
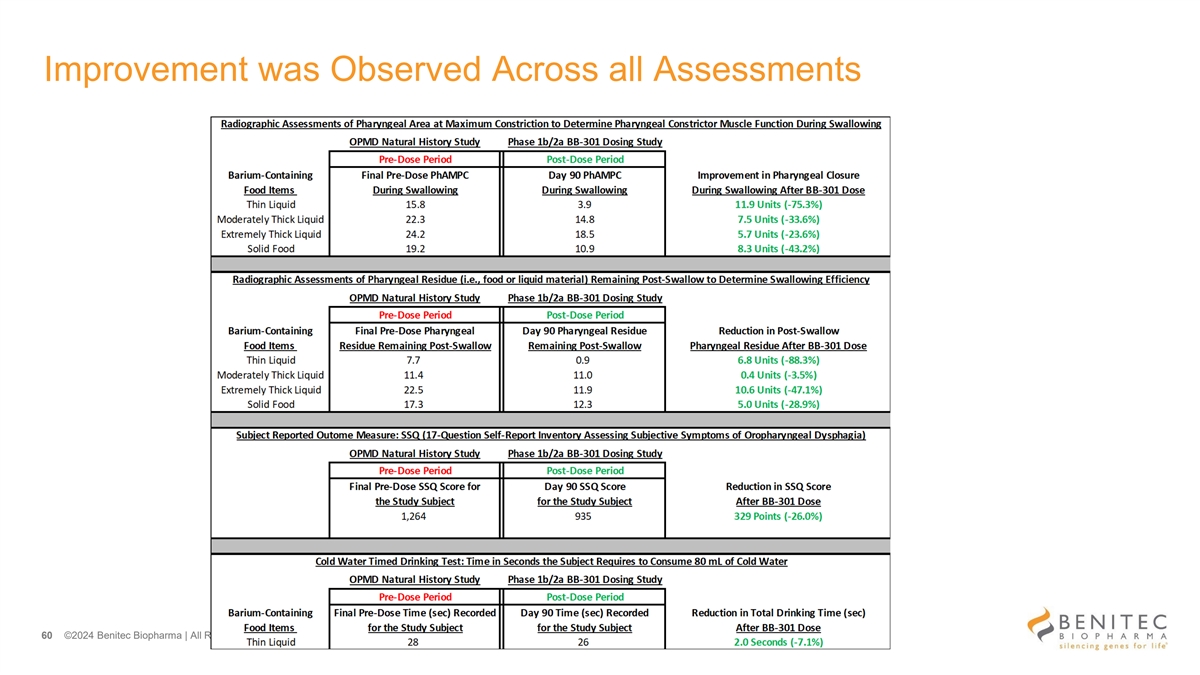
Improvement was Observed Across all Assessments 60 ©2024 Benitec Biopharma | All Rights Reserved

Preliminary Observations and Conclusions for Subject 1 • Subject 1 experienced progressive worsening of dysphagia during the pre-dose period as demonstrated by the videofluoroscopic swallowing study (VFSS) results, the cold water timed drink test results, and the subject-reported outcome results • Following the administration of BB-301, at the Day 90 time-point, Subject 1 demonstrated improvements in key clinical and videofluoroscopic assessments as compared to the average pre-dose assessments • The most significant VFSS improvements at Day 90 were observed for swallowing tasks centered on the evaluation of pharyngeal constrictor muscle function and swallowing efficiency in the context of the consumption of: − Solid foods (e.g., crackers) − Thick, non-solid foods (e.g., yogurt or pudding) − Thin liquids • The VFSS improvements observed for pharyngeal constrictor muscle function and swallowing efficiency in the context of the consumption of thin liquids, solid foods (e.g., crackers) and thick, non-solid foods (e.g., yogurt or pudding) correlated with a significant improvement in the key subject-reported outcome measure (i.e., Sydney Swallow Questionnaire) indicating an improvement in swallowing function as reported by Subject 1 61 ©2024 Benitec Biopharma | All Rights Reserved

Clinical Safety Update for the BB-301 Phase 1b/2a Clinical Study (NCT06185673) • The benign safety profile for Subject 1 (the first Study Subject dosed with BB-301) remains unchanged from that which has been reviewed by the Data Safety Monitoring Board • During the first week following the administration of BB-301, Subject 1 experienced heartburn • The heartburn was attributed to the prophylactic corticosteroids that are administered per Protocol to each Study Subject for Day -1 (the day preceding BB-301 dosing), Day 1 (the day of BB-301 dosing), and the 56 days following dosing • The heartburn was managed with a short course of prescription medication, and the heartburn resolved over the following three days • The use of a prescription medication to alleviate the heartburn renders the heartburn a Grade 2 Adverse Event 62 ©2024 B enitec B iopharm a | All R ights R es erved
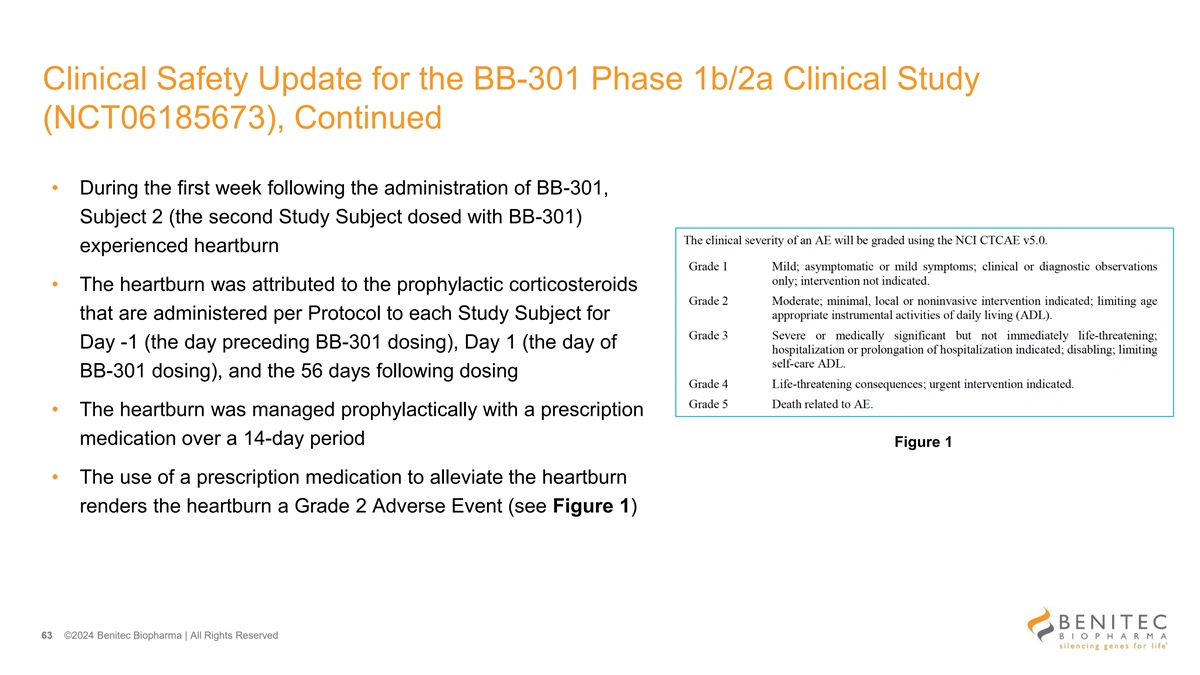
Clinical Safety Update for the BB-301 Phase 1b/2a Clinical Study (NCT06185673), Continued • During the first week following the administration of BB-301, Subject 2 (the second Study Subject dosed with BB-301) experienced heartburn • The heartburn was attributed to the prophylactic corticosteroids that are administered per Protocol to each Study Subject for Day -1 (the day preceding BB-301 dosing), Day 1 (the day of BB-301 dosing), and the 56 days following dosing • The heartburn was managed prophylactically with a prescription medication over a 14-day period Figure 1 • The use of a prescription medication to alleviate the heartburn renders the heartburn a Grade 2 Adverse Event (see Figure 1) 63 ©2024 Benitec Biopharma | All Rights Reserved

Clinical Safety Update for the BB-301 Phase 1b/2a Clinical Study (NCT06185673), Continued • According to the Phase 1b/2a Study Protocol, any Grade 2 Adverse Event not resolving within 14 days, assessed to be possibly related to the investigational product (see Figure 2) is characterized as a Dose Limiting Toxicity (DLT) and requires the expansion of the size of a Cohort from 3 Subjects to 6 Subjects • In this regard, the 14-day prophylactic management of the subject’s heartburn with a prescription medication triggers the expansion of the size of the Cohort 1 from 3 Subjects to 6 Subjects Figure 2 64 ©2024 B enitec B iopharm a | All R ights R es erved

Upcoming Milestones • Updates on additional subjects are anticipated later in 2024 • Updates on additional subjects at higher doses, and with longer durations of follow-up, are anticipated in 2025 • By year-end 2025, Benitec would potentially have clinical follow-up data for multiple study subjects for up to 12 months in Dose Cohort 1 and up to 9 months in Dose Cohort 2 • If clinical safety and efficacy data continue to evolve favorably over the first two dose cohorts, then, by early 2026 Benitec would plan to review the clinical data set with the FDA and inquire about plans for a pivotal study 65 ©2024 B enitec B iopharm a | All R ights R es erved

Appendix 66 ©2024 B enitec B iopharm a | All R ights R es erved
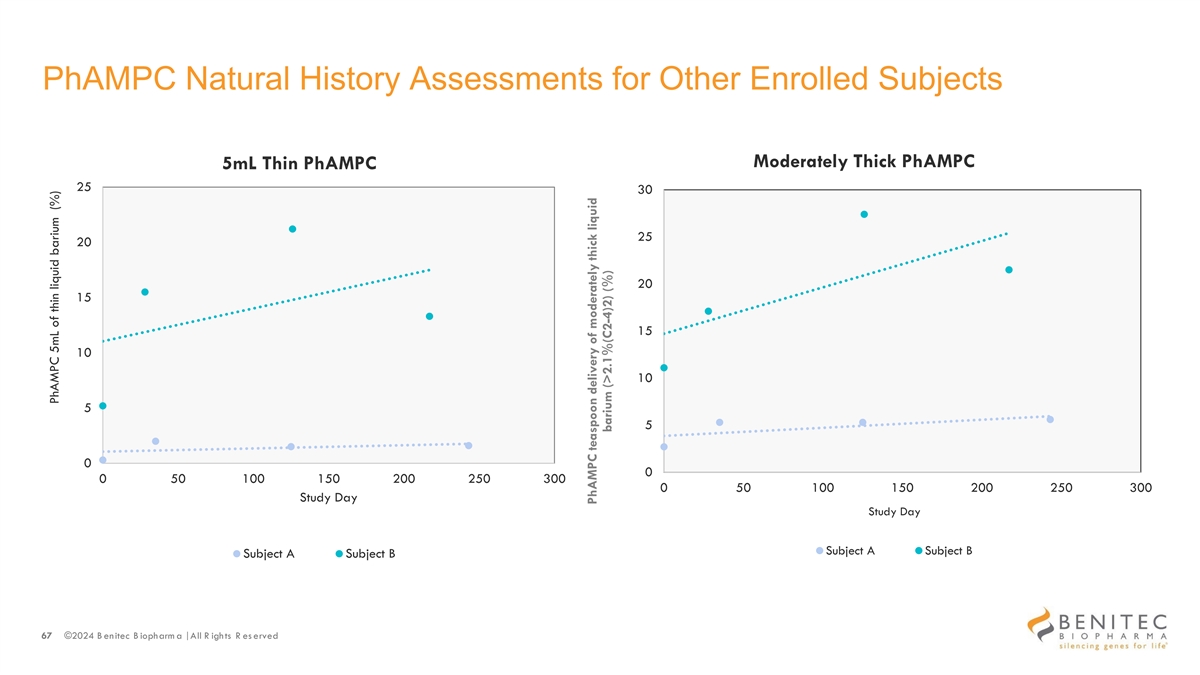
PhAMPC Natural History Assessments for Other Enrolled Subjects Moderately Thick PhAMPC 5mL Thin PhAMPC 25 30 25 20 20 15 15 10 10 5 5 0 0 0 50 100 150 200 250 300 0 50 100 150 200 250 300 Study Day Study Day Subject A Subject B Subject A Subject B 67 ©2024 B enitec B iopharm a | All R ights R es erved PhAMPC 5mL of thin liquid barium (%) PhAMPC teaspoon delivery of moderately thick liquid barium (>2.1%(C2-4)2) (%)
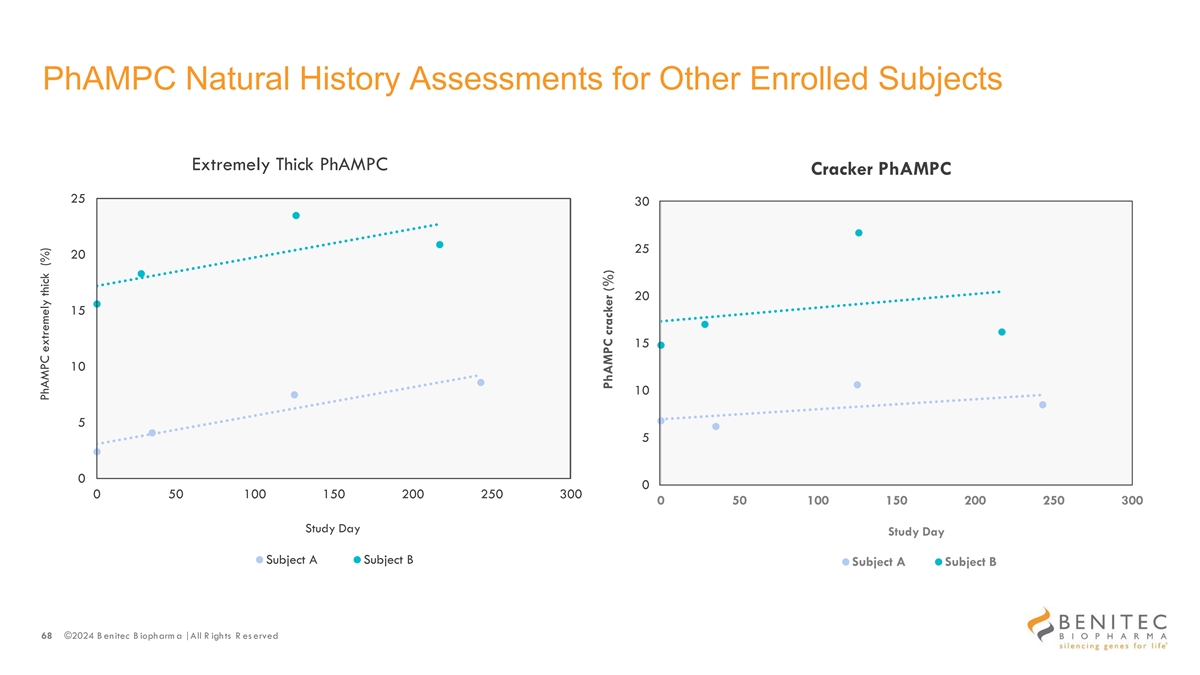
PhAMPC Natural History Assessments for Other Enrolled Subjects Extremely Thick PhAMPC Cracker PhAMPC 25 30 25 20 20 15 15 10 10 5 5 0 0 0 50 100 150 200 250 300 0 50 100 150 200 250 300 Study Day Study Day Subject A Subject B Subject A Subject B 68 ©2024 B enitec B iopharm a | All R ights R es erved PhAMPC extremely thick (%) PhAMPC cracker (%)
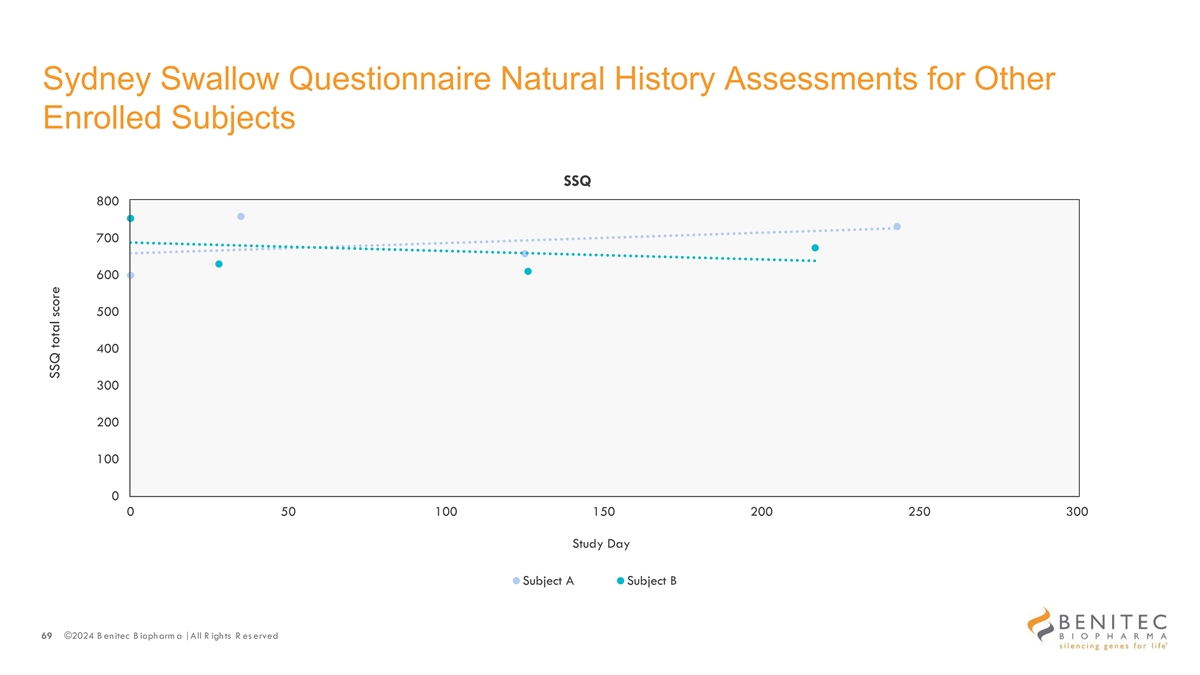
Sydney Swallow Questionnaire Natural History Assessments for Other Enrolled Subjects SSQ 800 700 600 500 400 300 200 100 0 0 50 100 150 200 250 300 Study Day Subject A Subject B 69 ©2024 B enitec B iopharm a | All R ights R es erved SSQ total score
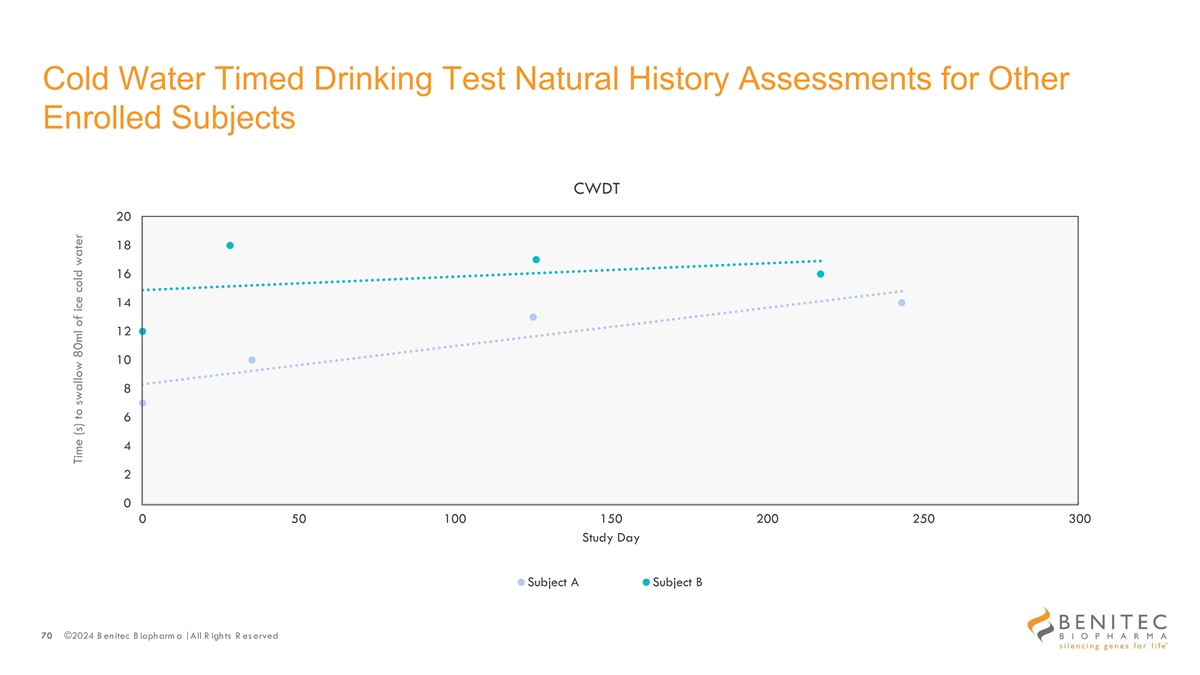
Cold Water Timed Drinking Test Natural History Assessments for Other Enrolled Subjects CWDT 20 18 16 14 12 10 8 6 4 2 0 0 50 100 150 200 250 300 Study Day Subject A Subject B 70 ©2024 B enitec B iopharm a | All R ights R es erved Time (s) to swallow 80ml of ice cold water

For additional information contact: Irina Koffler LifeSci Advisors, LLC 917-734-7387 ikoffler@lifesciadvisors.com 71 ©2024 Benitec Biopharma | All Rights Reserved